Longitudinal extensive transverse myelitis after COVID-19 vaccination (Spikevax mRNA-1273, Moderna) in a patient with rheumatoid arthritis
Article information
A 45-year-old male patient with rheumatoid arthritis visited the emergency department complaining of fever and urinary retention, which began 15 days after receiving his first dose of coronavirus disease 2019 (COVID-19) vaccination. He was treated with methotrexate 15 mg/week, hydroxychloroquine 200 mg/day, and sulfasalazine 1,000 mg/day. Neurological examination revealed bilateral leg weakness (Medical Research Council grade 3) and hypoesthesia below T11 dermatome. Spinal cord magnetic resonance imaging (MRI) showed high signal intensity lesions extending from medulla to L2 level (Fig. 1A). Cerebrospinal fluid (CSF) results were as follows: white blood cell 38/mm3, red blood cell 4/mm3, protein 123 mg/dL, and glucose CSF/serum 64/168 mg/dL. The CSF bacterial and virology, serum anti-myelin oligodendrocyte glycoprotein antibodies, anti-aquaporin receptor-4 antibodies, oligoclonal bands, and immunoglobulin G index were all negative. Acute transverse myelitis (ATM) was suspected, and intravenous methylprednisolone was administered for 5 days (1 g/day) followed by 21 days tapering course of oral prednisolone starting at 60 mg daily. Neurologic symptoms gradually improved 4 days after steroid administration and MRI repeated after 14 days of admission showed interval improvement (Fig. 1B). After 6 months, the patient was able to walk without assistance with mild hypoesthesia of both feet. There have been many reports of extensive ATM after different vaccinations [1]. Although previous reports showed focal ATM after administration of the Spikevax mRNA-1273 vaccine [2], our patient showed longitudinal extensive ATM with brainstem involvement. Autoimmune reactions between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein and tissue protein may result in central nervous system inflammation.
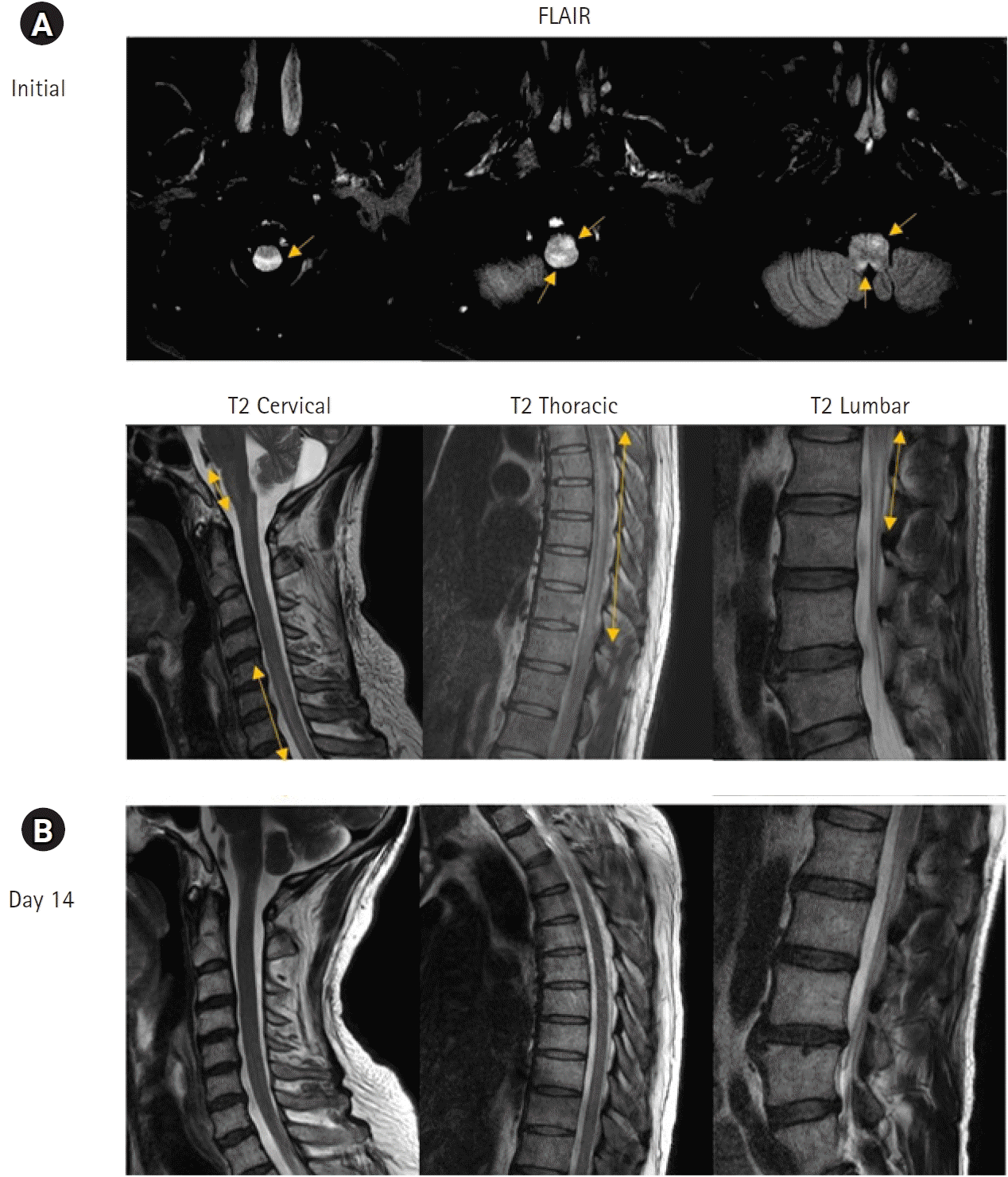
(A) Initial brain fluid-attenuated inversion recovery (FLAIR) axial view images and spinal cord T2-weighted sagittal view images show high signal intensity lesions (arrows) compatible with longitudinal extensive transverse myelitis. (B) Spinal cord T2-weighted sagittal view images show interval improvement of high signal intensity lesions after steroid pulse therapy.
Notes
Ethics statement
This study was approved by the Institutional Review Board of Hanyang University Hospital (No. 2022-10-020). Informed consent was waived by the Board.
Conflict of interest
No potential conflict of interest relevant to this article.
Author contributions
Conceptualization: YSK. Data curation: SC. Formal analysis: SC. Methodology: YSK. Project administration: YSK. Visualization: SC. Writing–original draft: SC. Writing–review & editing: YSK.
