Hypomagnesemia as a prognostic marker of ischemic stroke
Article information
Abstract
Background
Hypomagnesemia is associated with stroke severity and increased in-hospital mortality in patients with acute ischemic stroke. This study aimed to assess whether serum magnesium concentration could predict functional outcomes of patients with acute ischemic stroke.
Methods
A total of 1,006 patients with acute ischemic stroke were analyzed. A serum magnesium level <1.6 mEq/L was defined as hypomagnesemia. Poor functional outcome was defined as a 3-month modified Rankin Scale (mRS) score ≥4. Multivariate logistic regression models were used to determine the effect of hypomagnesemia on the prognosis of ischemic stroke. Furthermore, patients were grouped according to severity and type of stroke. Within each group, subgroup analyses and interaction analyses were performed to determine whether the effect of hypomagnesemia on functional outcomes was still valid under different clinical conditions.
Results
The adjusted odds ratio (OR) for poor 3-month mRS in patients with hypomagnesemia was 2.15 (95% confidence interval [CI], 1.16–3.98; P=0.015). Hypomagnesemia was significantly associated with poor 3-month functional outcomes in patients with minor stroke (Initial National Institutes of Health Stroke Scale [NIHSS] score <5: adjusted OR, 4.20; 95% CI, 1.67–10.59; P=0.002). A significant interaction (P=0.047) was also observed between hypomagnesemia and the severity of the initial NIHSS. Although there was no significant interaction (P=0.053), hypomagnesemia was significantly associated with poor functional outcomes in the cardioembolic stroke group (adjusted OR, 3.41; 95% CI, 1.24–9.41; P=0.018).
Conclusion
Hypomagnesemia was a strong prognostic marker of poor functional outcome in certain subgroups, especially in patients with mild stroke severity and cardioembolic stroke.
INTRODUCTION
Magnesium plays an essential role in numerous enzymatic processes, cellular metabolism, and neuronal function [1]. Although some studies have discovered the potential of magnesium as a neuroprotective agent [2-4], human trials have not demonstrated the efficacy of intravenous (IV) magnesium administration. In the Intravenous Magnesium Efficacy in Stroke (IMAGES) trial, IV infusion did not have a statistically significant effect 12 hours after stroke onset [5]. Additionally, in the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) trial, prehospital use of magnesium sulfate in acute ischemic stroke showed no improvement in disability outcomes at 90 days [6].
The effect of serum magnesium on the acute phase of ischemic stroke has also been studied. A prospective study has revealed that hypomagnesemia is associated with a higher incidence of ischemic stroke [7]. In addition, decreased serum magnesium levels at the time of admission can independently increase in-hospital mortality in patients with ischemic stroke [8]. Meanwhile, a higher serum magnesium concentration is related to a lower risk of stroke severity (National Institutes of Health Stroke Scale [NIHSS] ≥10) and mortality [9]. With increasing interest in the disability burden from ischemic stroke, we investigated the association between hypomagnesemia and functional outcomes expressed using the modified Rankin Scale (mRS). We also evaluated the interactions of hypomagnesemia with other risk factors, such as stroke severity, stroke type, age, and history of hypertension.
METHODS
Study population
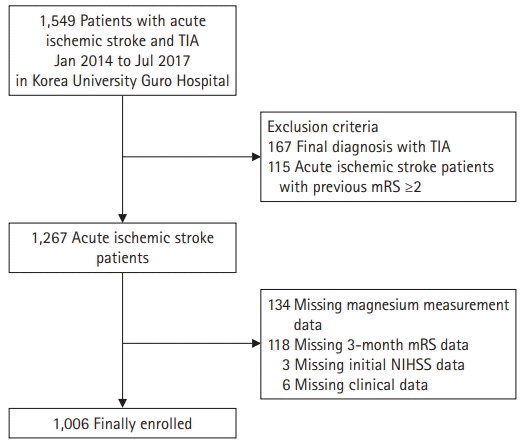
This was a single-center cross-sectional study. From January 2014 to July 2017, 1,549 patients with acute ischemic stroke or transient ischemic attack (TIA) who were admitted to the Department of Neurology of Korea University Guro Hospital were enrolled. The diagnosis of ischemic stroke was made based on the patients’ history, clinical manifestations, and neuroimaging results (computed tomography or magnetic resonance imaging) according to World Health Organization-defined criteria. The exclusion criteria were as follows: (1) diagnosis of TIA and (2) patients with acute ischemic stroke with previous mRS ≥2, excluding 282 patients from the study population. A total of 1,133 patients were included in this analysis. Furthermore, we excluded patients with missing data for the 3-month mRS, initial NIHSS score, serum magnesium concentration at admission, and additional clinical data such as body weight, height, serum triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) (flow chart of patient selection; Fig. 1). Finally, 1,006 patients were selected as the study population eligible for analysis.
Data collection
The following baseline information was collected: demographics of the patients, vascular risk factors, stroke severity (NIHSS score), laboratory test results, and stroke-related information. The demographic data of the patients included age, sex, weight, and height. Vascular risk factors for ischemic stroke include a history of hypertension, diabetes mellitus (DM), coronary heart disease, atrial fibrillation, and current smoking status. Information on these factors was gathered through interviews with the patient or the patient's family if the patient could not communicate. Current smoking status was defined as having smoked one or more cigarettes per day in the year before the onset of stroke. The demographics data of the patients and vascular risk factors obtained from the interviews were cross-matched with hospital records and laboratory data.
A series of routine laboratory investigations, including systolic blood pressure, diastolic blood pressure, total cholesterol concentration, serum TG concentration, serum HDL-C concentration, and serum LDL-C concentration, were performed. Blood pressure was measured when the patient arrived at the hospital. Blood samples used for all laboratory tests were taken within 24 hours of admission. Laboratory tests, including serum magnesium concentration, were performed in the Department of Laboratory Medicine, Korea University Guro Hospital. Serum magnesium was measured with an atomic absorption spectrometer using the xylidyl blue reaction, according to the manufacturer's instructions, at the Department of Laboratory Medicine, Korea University Guro Hospital.
Definitions and interpretation
Referring to previous studies, the criterion for hypomagnesemia was defined as <1.6 mEq/L (0.8 mmol/L) [10]. The putative cause of ischemic stroke was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification. The NIHSS score was used to describe the severity of the ischemic stroke. The initial NIHSS, defined as NIHSS, was documented by physicians as soon as the patient arrived at the emergency department before receiving specific treatment and was used for analysis. The mRS score was evaluated 3 months after discharge. The 3-month mRS was divided into two groups based on whether the patient was able to walk without assistance: good outcome (mRS <4) and poor outcome (mRS ≥4) [11].
Statistical analysis
Baseline demographical, laboratory data, and TOAST classification of the two groups were compared according to whether the serum magnesium level is less than 1.6 mEq/L or more than 1.6 mEq/L. Continuous variables were analyzed using the independent t-test and Mann-Whitney U-test, whereas the frequency of categorical variables was compared using the chi-square test. To assess the effect of hypomagnesemia on poor 3-month mRS scores, a multivariable logistic regression analysis was performed. Age, hypertension, DM, history of ischemic stroke, current smoking, and total cholesterol level were included as traditional prognostic factors for ischemic stroke. Potential confounders, such as the initial NIHSS score and cardioembolic stroke, were also included according to the researcher’s agreement. In addition, we performed an interaction analysis between hypomagnesemia and potential modifiers to assess whether the effect of hypomagnesemia on the 3-month mRS was affected by stroke severity (initial NIHSS score), cardioembolic stroke, age, or a history of hypertension. Subgroup analyses were performed using a multivariate-adjusted model stratified by initial NIHSS score (<5 vs. ≥5), whether the type of ischemic stroke was cardioembolic, age (<65 vs. ≥65 years), and history of hypertension. To assess unmeasured confounding effects [12] E-values were calculated for the odds ratios (ORs) of hypomagnesemia in the minor and cardioembolic stroke subgroups. All P-values were two-tailed with a significance level of 0.05. All statistical analyses were performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA).
RESULT
First, the baseline characteristics of 1,006 patients were analyzed. The mean age of the study population was 66±12.9 years. Of these, 651 (64.7%) were male. The median initial NIHSS score was 3 (interquartile range, 1–6). The number of patients with poor functional outcome (3-month mRS ≥4) was 149 (14.8%). The baseline characteristics of the patients divided into two groups according to serum magnesium levels are presented in Table 1. The proportion of men was lower in patients with hypomagnesemia (<1.6 mEq/L) than in patients with higher serum magnesium levels (≥1.6 mEq/L). Patients with hypomagnesemia had significantly more conventional risk factors such as hypertension, DM, and atrial fibrillation. Regarding laboratory data, patients with hypomagnesemia had lower total cholesterol and LDL-C levels. Lastly, in the hypomagnesemia group, 27.8% of ischemic stroke cases were due to cardioembolism, while only 16.3% had a cardioembolic stroke in the other group.
Second, the effect of hypomagnesemia on the functional outcomes of ischemic stroke was estimated using univariate and multivariate logistic regression analyses (Table 2). When analyzed using a univariate logistic regression model, the odds of poor 3-month mRS were 2.29-fold higher in patients with hypomagnesemia than in those without hypomagnesemia (95% confidence interval [CI], 1.45–3.62; P<0.001). With adjustment, hypomagnesemia was significantly associated with an increased risk of poor 3-month functional outcomes compared with higher serum magnesium levels (OR, 2.15; 95% CI, 1.16–3.98; P=0.015). Age, hypertension, and initial NIHSS score were also significantly associated with poor 3-month mRS scores.
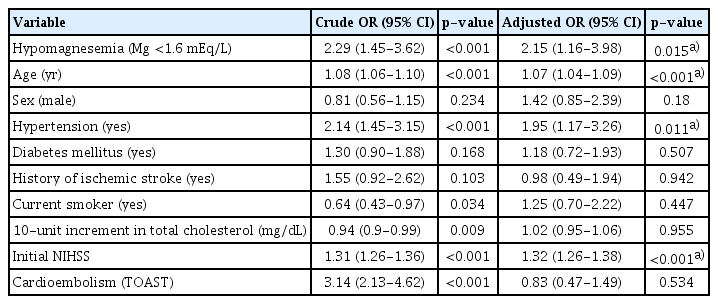
Crude and adjusted ORs of poor 3-month mRS using univariate and multiple logistic regression analyses
Third, subgroup and interaction analyses were performed to assess the effects of hypomagnesemia on functional outcomes in different clinical situations (Table 3). The patients were divided into two groups according to the independent variables mentioned above: minor (NIHSS score <5) and moderate-severe (NIHSS score ≥5) stroke, age <65 and ≥65 years, and presence of hypertension. Although cardioembolism was not an independently significant variable in the adjusted analysis, it was considered because patients with hypomagnesemia had more atrial fibrillation than those without hypomagnesemia [13] presumed to have a confounding relationship with serum magnesium level. In the analysis of the four groups divided by initial NIHSS and magnesium levels, the difference between the ORs of the two magnesium groups was more significant in the minor stroke group (adjusted OR, 1[ref] to 4.20) than in the moderate-severe stroke group (adjusted OR, 16.27–21.58). This is further supported by a significant interaction between hypomagnesemia and the severity of the initial NIHSS score (P=0.047). The E-value of the minor stroke/hypomagnesemia group was 7.87 for the point estimate and 2.73 for the CI. Similarly, hypomagnesemia was significantly associated with poor functional outcome only in the cardioembolic stroke group (adjusted OR, 3.41; 95% CI, 1.24–9.41; P=0.018). The E-value was 6.28 for the point estimate and 1.79 for the CI. The interaction analysis between hypomagnesemia and cardioembolism showed a tendency toward significance (P=0.053). Furthermore, the association between hypomagnesemia and poor 3-month mRS was significant in patients with a history of hypertension (adjusted OR, 4.42; 95% CI, 1.99–9.80; P<0.001) and in older patients (≥65 years) (adjusted OR, 8.11; 95% CI, 3.38–19.48; P≤0.001).
DISCUSSION
In our study, hypomagnesemia was associated with a higher risk of poor 3-month mRS scores, suggesting the role of magnesium as a prognostic marker in acute ischemic stroke. Furthermore, hypomagnesemia showed a stronger relationship with poor functional outcomes in certain subgroups of ischemic stroke, especially mild stroke and cardioembolic stroke.
In moderate-to-severe stroke, the initial severity of the stroke is estimated to have a major contribution to the outcome [14]. However, in minor stroke, clinical situations, such as early neurological deterioration, are related to the outcome [15], and together with other risk factors, the serum magnesium level could affect the course of the disease through several physiological actions. Hypomagnesemia deteriorates endothelial stability by inducing oxidative stress and promoting an inflammatory response [16]. Moreover, in a study of coronary artery disease, hypomagnesemia was shown to increase thrombus formation [17]. In addition, magnesium is involved in local blood pressure regulation by inducing vasodilatation [18]. In this context, hypomagnesemia could aggravate the ischemic lesion itself and perhaps the collateral circulation, thus contributing to neurological deterioration in patients with minor stroke.
Among the subtypes of ischemic stroke, cardioembolic cerebral infarction has a poor prognosis [19-21]. Our results showed that patients with cardioembolic stroke, particularly those with low serum magnesium levels, had worse outcomes. Although the effect of serum magnesium concentration on the prognosis of cardioembolic stroke has not been fully established, its effects on cardiac function have been well studied [13]. As an essential cofactor for the Na-K ATP pump, magnesium is involved in the transport of sodium and potassium across the cell membrane [22]. Dysfunction of this pump in a low magnesium environment can disturb the regulation of myocardial excitability. A previous study showed that low magnesium concentration could increase sinus node automaticity [23]. Hypomagnesemia can also increase the dose required to control the rate of atrial fibrillation [24]. Since hypomagnesemia induces and exacerbates atrial fibrillation, it can also increase the frequency of early recurrent embolization, which is one of the most important factors that worsen cardioembolic stroke [25]. According to a recent study, hypomagnesemia also negatively influences ischemic lesions in the acute stage. In this study for patients who received IV thrombolytic therapy, patients with hemorrhagic transformation (HT) have significantly lower serum magnesium levels than those without HT [26]. Since HT is inherently more frequent in cardioembolic stroke, concomitant hypomagnesemia worsens the prognosis.
This study had some limitations. First, as this was an observational cross-sectional retrospective study, a causal relationship between hypomagnesemia and a poor prognosis of ischemic stroke could not be established. The study population was selected based on inclusion criteria during recruitment, and follow-up of patients after discharge was carried out thoroughly. Second, this study was conducted in a single center. Therefore, multiple center-based studies are needed to confirm the association between hypomagnesemia and functional outcome of ischemic stroke in the general population.
Our study demonstrates the diagnostic utility of hypomagnesemia in predicting poor functional outcomes in patients with acute ischemic stroke. In addition, the results were particularly pronounced in specific patient groups, such as those with minor and cardioembolic strokes. In future studies with magnesium as a neuroprotective agent, it may be necessary to design a more specific patient population.
Notes
Ethics statement
The study protocol was approved by the Institutional Review Board of the Korea University Medical Center, Guro Hospital (IRB No. 2011GR0218). The requirement of informed consent was waived.
Conflict of interest
No potential conflict of interest relevant to this article.
Author contributions
Conceptualization: SYA, CKK. Data curation: DWL, SHK, HR. Formal analysis: HR, CKK, DWL, SHK. Methodology: CKK, SYA, HR. Project administration: KMO. Visualization: SHK, DWL, JHH. Writing–original draft: HR, CKK, SYA. Writing–review & editing: HJK, JHH, CKK, KMO.