Neuroleptic malignant syndrome cases in a Moroccan intensive care unit: a retrospective analysis and literature review
Article information
Abstract
Background
Neuroleptic malignant syndrome (NMS) is a rare but potentially life-threatening neuropsychiatric emergency. The aim of our study was to update our bedside procedures by investigating NMS cases managed in the intensive care unit (ICU).
Methods
This retrospective study included all NMS patients admitted to our hospital between January 2012 and December 2019. The variables analyzed included demographics, diagnosis, therapeutics, and outcomes.
Results
This study included 20 patients, with an average age of 36.6 years. The male to female ratio was 1:4. No patient had a history of NMS, and 60% of the patients had schizophrenia. First-generation neuroleptics (NLs) were the most commonly prescribed drugs (80%). The mean time between the introduction of NLs and onset of symptoms was 7.6 days. Rigidity was observed in 90% of the patients, hyperthermia and neuropsychic syndrome in 65%, and dysautonomia in 50%. The creatine phosphokinase level in all patients was four times the normal value. Mechanical ventilation was required in 20% of the patients and hemodialysis in one patient. None of the patients received specific therapy. The mean duration of ICU stay was 10 days. The mortality rate was 10%,, mainly associated with renal failure. The analysis of the predictors of mortality was limited by the size of our cohort.
Conclusion
NMS is a rare condition requiring multidisciplinary implementation of contextualized and updated procedures. Early detection and supportive treatment could improve the prognosis in resource-limited settings, where specific treatments are not available. Predictive risk factors should be investigated in larger multicenter cohorts.
INTRODUCTION
Neuroleptic malignant syndrome (NMS) is an idiosyncratic reaction mainly related to the use of antipsychotic agents (first-or second-generation neuroleptics [NLs]). It is characterized by a myriad of clinical signs, including altered mental status, muscular rigidity, hyperthermia, and dysautonomia. This life-threatening neuropsychiatric emergency is rare, with an estimated incidence of 0.2% among NL users [1], and requires early therapeutic management and intensive care for potentially severe presentations. Mortality, caused by dysautonomic and systemic complications (infections, venous thromboembolism, rhabdomyolysis, acute renal failure, respiratory failure, etc.), decreased by 76% since the first reports in the 1960s; the current mortality rate is estimated at 10%–20% [2,3]. This is indicative of greater awareness, earlier diagnosis in both emergency settings and psychiatric wards, and interventions that are more aggressive than before. Since NMS requires a high degree of clinical suspicion for diagnosis and treatment, itis rightly a syndrome more often considered in differential diagnosis than is actually diagnosed. Moreover, considering its low incidence, there is limited evidence in the literature. Although the management of NMS is mostly performed in critical care settings, the issue is either not addressed or poorly addressed in the guidelines of the international and national scientific societies of intensive care and emergency medicine. This study aimed to analyze the NMS cases in our hospital to improve clinical decision-making based on the updated and contextualized bedside procedures.
METHODS
Study design and setting
This retrospective, observational, monocentric study evaluated patients aged ≥16 years who were admitted to our intensive care unit (ICU) between January 2012 and December 2019. The diagnosis of NMS was based on the diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) of the American Psychiatric Association [4] (Table 1). The diagnosis of NMS was made based on the presence of exposure to a dopaminergic antagonist within the last 72 hours, a suggestive symptomatology, and negative examination results for infectious, toxic, metabolic, and neurologic causes. The study setting was a 14-bed medico surgical adult ICU in a tertiary university hospital in Morocco.
Data collection
Study data were collected retrospectively from both paper charts and electronic medical records of patients using HOSIX (SIVSA Soluciones Informáticas, Vigo, Spain) electronic data capture tools present at our university hospital. The variables analyzed included demographic characteristics, patient’s history and comorbidities, all drugs involved, diagnostic parameters, therapeutics, and evolution.
Statistical analysis
Statistical analysis of the parameters was performed using IBM SPSS ver. 20 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to summarize the baseline patient characteristics. The results are expressed as numbers and percentages for qualitative variables and as means±standard deviations for the quantitative variables. The quantitative and qualitative variables were compared using the two-sample t-test derived from Student t distribution and Fisher’s exact test based on the N-1 chi-square test through univariate analysis. The statistical significance threshold was set at a P-value of 0.05. Analysis of predictive factors of mortality through multivariate analysis was not performed because of the limited number of patients in the “death” group, i.e., the group with deceased patients.
RESULTS
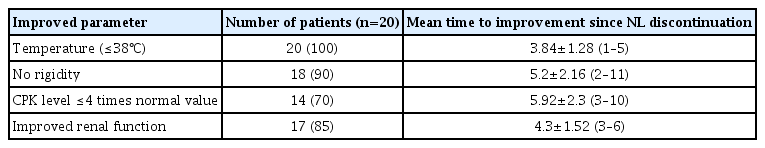
This study included 20 patients (16 males and 4 females) from the 6,090 patients admitted to our ICU between 2012 and 2019, with a mean age of 36.6±19.5 years (range, 18–84 years). The underlying comorbidities in our population were schizophrenia (60%), bipolar disorder (20%), substance dependence (25%), mental retardation (10%), acute psychosis (10%), dementia (5%), and delirium (5%). None of the patients had a history of NMS. There were no other medical comorbidities, except for toxic addictions (smoking and cannabism) in five patients and psychomotor disability in two patients. In all the patients, NMS was caused due to the use of antipsychotic drugs. First-generation conventional NLs were the most commonly used drugs (80% of the patients) and mainly administered along with atypical NLs or other drugs (70% of the patients). Atypical NLs were used in 11 patients (55%). The medications used in our population included first-generation conventional NLs (haloperidol, 40%; levomepromazine, 35%; and chlorpromazine, 35%), second-generation atypical NLs (risperidone, 25%; olanzapine, 15%; and amisulpride, 10%), and other drugs (selective serotonin reuptake inhibitors [SSRIs], benzodiazepines, and anticholinergics). No case of dopaminergic agonist withdrawal was reported. The parenteral mode of administration was used in only four cases, and 10% of the patients required a rapid increase in NL doses. The mean time between the introduction of NLs and the onset of symptoms was 7.6±7.1 days (range, 0–30 days). The mean time between symptom onset and hospital admission was 1.3±1.5 days (range, 0–5 days). Rigidity was observed in 90% of patients, hyperthermia and neuropsychic syndrome in 65%, and dysautonomia in 50%. All patients presented a creatine phosphokinase (CPK) level four times more than the normal value. The average CPK was 4,810±9,789 IU/L, with extremes ranging from 626 to 42,670 IU/L. The average Sachdev rating scale score on admission was 11±3.09 (range, 3–14). Examinations for infectious, toxic, metabolic, and neurologic causes were negative in all patients. The distribution of patients according to different diagnostic criteria is presented in Table 2. Psychotropic treatment was stopped in all patients as soon as NMS diagnosis was suspected. Mechanical ventilation was required in 20% of the patients for a mean duration of 14.75 days (range, 1–28 days). Tracheotomy was performed in two patients on days 4 and 13 of intubation, respectively. All patients underwent respiratory physiotherapy and fluid resuscitation. A continuous intravenous infusion of nicardipine at a dose of 2–6 mg/hr was required in one patient. One patient needed catecholamines, while another needed hemodialysis. Diazepam, midazolam, or clonazepam against agitation were required in 25% of the patients. Contention was necessary in one patient. No patient was administered dantrolene or bromocriptine, and none received electroconvulsive therapy (ECT). The mean duration of ICU stay was 10±15 days (range, 1–67 days). The overall outcome was favorable in 90% of the patients. The improvements in temperature, rigidity, CPK levels, and renal function varied (Table 3). One case progressed to persistent acute renal failure, classified as Risk; Injury; Failure; Loss; End stage kidney disease (RIFLE) 3. One case of cerebral hemorrhage due to ruptured arteriovenous malformation was recorded. The reintroduction of atypical NLs administered at low doses was conducted after a 15-day therapeutic interval in six patients (30%). No adverse effects were observed. The mortality rate was 10%, and the deaths were associated with renal failure complications. Two patients developed metabolic arrhythmias, mainly induced by refractory hyperkalemia and exacerbated by severe rhabdomyolysis. The mean time from ICU admission to death was 4±1.41 days (range, 3–5 days). When comparing the “death” (n=2) and “recovery” (n=18) groups, univariate analysis identified nine variables that were significantly (P<0.05) associated with mortality in NMS in our population (Table 4). The analysis of predictive factors of mortality through multivariate analysis was not possible given the limited number of patients in the “death” group.
DISCUSSION
This study, comprising 20 cases, is one of the largest cohorts of NMS patients in Morocco and one of the few studies conducted in ICUs. The incidence of NMS among NL users varies between 0.024% and 3% [5]. This is difficult to assess accurately due to population heterogeneity, variability in diagnostic criteria, and the methodological limitations of retrospective studies. In our study, NMS was predominant in young adult males, as similarly reported in the literature [6]. This can be explained by the higher skeletal muscle mass in males, and therefore more visible symptoms and more severe forms [7]; higher frequency of schizophrenia in males, and therefore a higher need for antipsychotics at elevated doses [8]; and sexual dimorphism in dopaminergic pathways, as suggested in recent studies [9].
NMS was first described in 1960 with haloperidol and was labeled as malignant by the Parisian group of the Sainte-Anne Hospital, analogous with the malignant syndrome of infectious diseases widely prevalent in the 1950s and 1960s [10]. However, any drug interfering with dopaminergic transmission can lead to NMS [11]. Combination of antipsychotic drugs, combined use of an antipsychotic with lithium or carbamazepine, abrupt discontinuation of a dopaminergic agonist such as levodopa in Parkinson disease patients, and the use of antiemetics such as metoclopramide have been reported to induce NMS. The combined use of several therapeutic classes of drugs seems to be associated with a higher risk of NMS, despite the higher affinity of conventional antipsychotics for dopaminergic D2 receptors [12,13]. In our series, no cases of dopaminergic agonist discontinuation were reported, and conventional NLs often combined with atypical NLs were most commonly used. Recently, more researchers have suggested “malignant extrapyramidal autonomic syndrome” diagnosis instead of NMS [14] to improve proactive screening in the absence of the use of antipsychotic drugs. The risk of NMS is higher within the first month of treatment at high doses, especially when administered parenterally or after a rapid dose change. Physical restraint during psychomotor agitation is often associated with high titration rates and parenteral therapies, and therefore increases the risk of NMS [15]. In our population, NMS occurred within a mean interval of 7 days (range, 24 hours–30 days), all doses were standard, and the parenteral route was only used in 20% of the cases. Therefore, NMS can occur at any time during treatment, even with standard doses, and regardless of the route of administration. Other risk factors have been reported, such as advanced age, comorbid medical conditions, mental retardation, history of NMS, and personal and/or family history of catatonia [16].
Regardless of the trigger mechanism (dopaminergic antagonism, dysautonomia, direct muscle toxicity of NL, etc.), the physiopathology of NMS is complex and involves a cascade of dysfunctions in multiple neurochemical and neuroendocrine systems, leading to end-stage hypermetabolic syndrome [8,17-19]. NMS is classically [1] characterized by four cardinal signs: hyperthermia, muscular rigidity, dysautonomia, and altered mental status. Neuropsychic syndrome usually precedes the systemic symptoms. Muscle rigidity can be generalized and could be symmetric (opisthotonos) or focal (blepharospasm, oculogyric crisis, and trismus). NMS hyperthermia usually presents high body temperatures, with no major peaks or fluctuations, no shivering, and unresponsiveness to conventional antipyretics. However, the clinical presentation of NMS can be heterogeneous and challenging (Table 5) [13,20-23]. Cases of NMS without muscle rigidity have been reported in the literature [24,25] as well as in this study (10% of the sample). Other extrapyramidal motor symptoms, such as tremor, chorea, akinesia, mutism, dysarthria, dysphagia, and other dystonic movements, may guide the diagnosis, but they are inconstant and non-specific. Approximately 20% of the patients had dysphagia or mutism, while hyperthermia was reported in only 65%. Apyretic NMS cases can be explained by the early diagnosis of NMS and/or a delayed onset of hyperthermia when compared to early symptoms such as rigidity [21]. Non-typical presentations are mostly associated with atypical NLs [26]. This is consistent with our study findings, where in the absence of rigidity and hyperthermia were observed respectively in 25% and 50% of the cases using atypical NLs compared to 0% and 33.3% of the cases using conventional NLs.
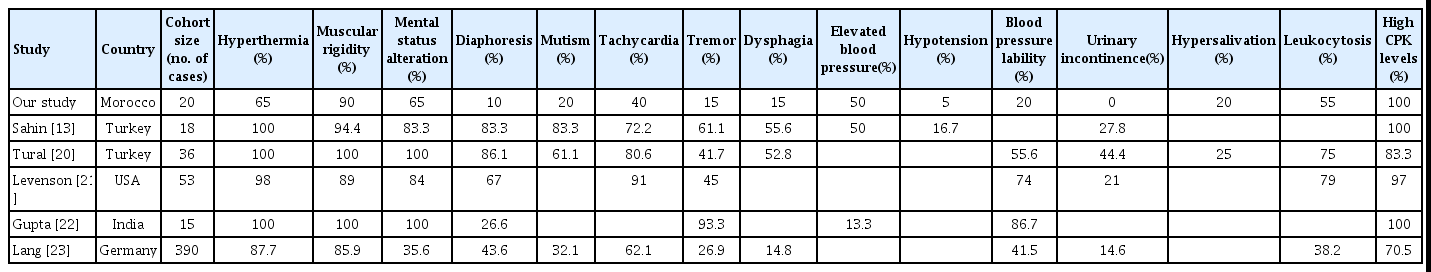
Heterogeneity of the clinical-biological presentation of neuroleptic malignant syndrome in different studies
Although no biomarkers are specific, laboratory assessment is necessary to support the diagnosis of NMS by excluding other diagnoses and to assess complications. Rigidity and hyperthermia lead to muscle damage and rhabdomyolysis (elevated CPK levels and myoglobinemia) with a risk of hyperkalemia, hyperphosphatemia, hyperuricemia, hypocalcemia, heart rhythm disturbances, disseminated intravascular coagulation, and renal failure [27]. The CPK level is considered both a diagnostic and prognostic marker of NMS (especially if CPK >1,000 IU/L), as well as a monitoring factor to assess the course and effectiveness of the treatment (kinetics). A mild or no increase in the CPK level may be idiopathic or related to the early stages of NMS, where rigidity is absent or poorly developed and maybe associated with physical restraint and intramuscular injections, especially in catatonic psychotic patients. Leukocytosis with or without inversion of the formula is also common because of sympathetic hyperactivation even in the absence of infection [27].
The 2017 study by Gurrera et al. [28] showed that the 2011 consensus diagnostic criteria [29] incorporated in DSM-5 were superior to those of DSM-IV [30] but required clinical validation. The DSM-IV, Nierenberg scale, and Sachdev rating scale confirmed the diagnosis of NMS in only 55%, 65%, and 85% of our cases, respectively (Table 2). The DSM-5 consensus (Table 1) [4] does not require major and minor criteria and seems more likely to identify non-typical NMS presentations. It seemed appropriate to base the NMS diagnosis on the DSM-5 criteria in our daily practice. NMS is an exclusion diagnosis, even with well-defined diagnostic criteria [11]. Patients taking NLs may present a pathological condition unrelated to their usual drug use, and some differential diagnoses are potentially life-threatening, including meningoencephalitis, epilepsy, toxic or septic encephalopathy, malignant hyperthermia, heat stroke, serotonin syndrome, and malignant catatonia [31]. Brain scan, lumbar puncture, toxicological screening, electroencephalogram, and metabolic and infection testing may be necessary to rule out disorders that are more critical. Some differential diagnoses are less known to intensivists and require expertise and multidisciplinarity. In fact, NMS can easily be confused with other dysautonomia diseases (serotonin syndrome, malignant catatonia, or clozapine-induced hyperthermia) where rigidity, hyperpyrexia, dysautonomia, and polymedications are common. In our series, SSRIs were used in 15% of the cases at non-toxic doses in combination with NLs. Serotonin syndrome is related to selective toxicity of SSRIs but is characterized by tremor, hyper-reflexia, myoclonus, ataxia, more gastrointestinal symptoms (diarrhea, nausea, and vomiting), less rigidity, and hyperthermia [32]. Malignant catatonia is characterized by prodromal behavioral symptoms (psychosis, agitation, and catatonic excitement) and motor symptoms (dystonia, waxy flexibility, and repetitive stereotyped movements). Because catatonia symptoms are present in NMS too, distinguishing between the two is difficult [23]. Clozapine-induced hyperthermia, a known and variable side effect of clozapine, is considered a clinical presentation of NMS by some authors [33].
The severity of NMS is associated with the onset of life-threatening complications related to the physiopathology of the disease, long ICU stay, and immobility [3,31]. The Sachdev rating scale [34] allows for the diagnosis of NMS, severity assessment, and follow-up. A total score >8 and a score ≥2 in at least three of the six categories establish the diagnosis of NMS. Other complications not considered in the Sachdev rating scale are addressed in the organ failure scores usually used in intensive care and emergency settings, i.e., the quick-Sequential Organ Failure Assessment (SOFA) and SOFA scores. The quick-SOFA score is an easily reproducible clinical score in an emergency setting with a significant prognostic value. It includes three items: hypotension (systolic blood pressure ≤100 mmHg), high respiratory rate (≥22 breaths/min), and altered consciousness (Glasgow coma scale score ≤14). The SOFA score is used for the severity assessment and follow-up of critically ill patients in daily practice. The Sachdev rating scale requires further clinical validation in various populations and more demanding assessment, but it could be an interesting follow-up marker in the ICU setting.
Treatment must be initiated as soon as NMS is suspected [31]. These patients require monitoring and intensive care, which cannot be provided in the psychiatric ward. Both delayed diagnostic and therapeutic management and inadequate management settings have been recognized as the prognostic factors for morbidity and mortality [3]. This observation in our practice has led to a close collaboration between the two departments of psychiatry and emergency/intensive care that involves emergency care training for psychiatrists, multidisciplinary meetings, and rigorous transfer regulation of suspected cases of NMS. Considered a prognostic factor [3], the causal psychotropic treatment was discontinued in all our patients as soon as NMS was suspected. Resuscitation measures and specific therapies are shown in Table 6 [3,31,35,36]. Given the rarity of NMS and the acute nature of its onset, the current recommendations are based on a low level of evidence. Randomized controlled trials are lacking, and the main treatment guidelines are based on case studies, meta-analyses, or expert opinions [36]. In addition, recommendations must be adapted to the local specificities. Dantrolene [37,38] is a muscle relaxant antagonist of the ryanodine-1 receptors of striated muscles and is recommended in hypermetabolic forms of NMS (hyperthermia and rigidity). None of our patients was administered dantrolene, as it was unavailable. The central active dopaminergic agonists (bromocriptine, amantadine, or levodopa) have been reported to reduce the recovery time and mortality [38]; however, these molecules are used outside marketing authorization, and the treatment duration is not defined. ECT stimulates serotoninergic and dopaminergic neurotransmission and is currently the gold standard in cases non-responsive to specific pharmacological treatments [11,36]. However, the following imperatives need to be clarified: definition of pharmacological treatment failure, electrode positioning, and the intensity, frequency, and duration of sessions. Current guidelines recommend 6–10 bilateral ECT sessions [39]. It is performed under general anesthesia, and its requirements include pre-anesthetic assessment, optimal anesthetic platform, vigilance, and anesthesiologist-operator communication. Some anesthetic agents should be used with caution. Sevoflurane, succinylcholine, or their combination is associated with malignant hyperthermia; hence ,they should be avoided. The current availability of sugammadex makes rocuronium an interesting alternative to succinylcholine [40]. ECT is performed a tour center under sedation without intubation, but none of our patients received it as a treatment for NMS.
The reintroduction of psychotropic treatment in patients who need it but are at a risk of NMS recurrence is a real dilemma for clinicians. Recurrence risk is unpredictable, and relapse rates are highly variable [41]. When reintroduction is considered, the following recommendations [2,42] are essential to minimize the recurrence risk without canceling it: interval of at least 2 weeks, complete resolution of symptoms, use of different and less powerful molecules, avoiding lithium and parenteral therapies, slower titration schemes, prevention of dehydration, and close monitoring. Long-term ECT appears to be an interesting alternative. In our study, the reintroduction of NLs was performed in six patients (30%), using atypical molecules at low doses after a therapeutic interval of 15 days. No adverse events were reported.
Mortality rates in NMS range from 5% to 20%; death occurs most often in the course of multivisceral failure secondary to complications of NMS, mainly renal [2,3,13,43]. The main prognostic factors observed are acute renal failure, respiratory failure, sepsis, advanced age, and CPK levels [2,3,13,44]. The mortality rate in our study was 10%, and the deaths were related to rhabdomyolysis and renal failure. Advanced age, renal, hepatic, and hematological failure, as well as mechanical ventilation were significantly associated with mortality in our study based on the univariate analysis. Daily assessment of the SOFA score should be performed, and treatment that is more aggressive should target elderly and frail patients, especially those with limited physiological and nephronic reserves. Most episodes usually resolve within 2 weeks, but prolonged cases with residual catatonia and motor signs have been reported [42]. Recovery was longer in one of our cases, as it was complicated by meningeal hemorrhage [45]. Risk factors for the worsening of NMS include the use of conventional antipsychotic drugs and the presence of underlying structural brain pathologies. Most patients do not develop neurological sequelae, except in cases of severe hypoxia or prolonged hyperthermia [42]. This implies the importance of temperature control and optimization of oxygenation in NMS patients. These patients are considered to have brain injury, and management of secondary cerebral systemic aggressions is necessary. Interestingly, our patients’ outcomes are consistent with those reported in the literature, and no “antidotal” therapy was given to any patient. This highlights the importance of early diagnosis and supportive treatment in resource-limited settings where specific treatments are not available. However, early initiation of specific treatment, if available, may have affected the outcome of the deceased patients. Analysis of the predictive factors of mortality was not possible given the limited number of patients in the “death” group; however, through univariate analysis, this study identified the important complications that require close monitoring. The stated conclusions need to be verified using larger samples in future multicentric studies. Another limitation of the retrospective nature of this study is the lack of long-term follow-up of all the patients.
In conclusion, NMS is a diagnostic and therapeutic neuropsychiatric emergency. It requires clinical, multidisciplinary, and dynamic expertise to avoid overlooking atypical forms or differential diagnoses. The use of more flexible diagnostic criteria is essential to detect atypical forms, which are more frequent with second-generation antipsychotics and non-psychotropic drugs. The identification of prognostic factors specific to our context could improve the management, but this would require large national multicentric cohorts for research. Nevertheless, advanced age, high CPK levels, and renal failure are the potential factors to be considered in future studies, and they require the clinician’s full consideration during management. Finally, this study allowed us to update and contextualize our bedside procedures (Supplementary Material 1).
Notes
Ethics statement
This study was approved by the local Institutional Review Board of Comite d’Ethique Hospitalo-Universitaire de Fes (IRB No. 16/21), and the need for informed consent from patients was waived.
Conflict of interest
No potential conflict of interest relevant to this article.
Author contributions
Conceptualization: ST, MH, NH. Data curation: ST, MH, YYK. Formal analysis: ST, MH. Methodology: ST, NH. Project administration: ST, NH, NK. Visualization: ST, AE, BB. Writing–original draft: ST. Writing–review & editing: all authors.
Supplementary materials
Supplementary materials can be found via https://doi.org/10.18700/jnc.210019.