The good genotype for clopidogrel metabolism is associated with decreased blood viscosity in clopidogrel-treated ischemic stroke patients
Article information
Abstract
Background
Blood viscosity (BV) is a measurement of the intrinsic resistance of blood to flow, and high BV increases thromboembolic risk. Although laboratory documentation of clopidogrel resistance has been shown to predict an increased risk of cardiovascular events in patients with ischemic stroke, there is no evidence that cytochrome P450 2C19 (CYP2C19) polymorphisms in clopidogrel-treated patients influence BV after ischemic stroke.
Methods
Patients with ischemic stroke or transient ischemic attack within 7 days of symptom onset from April 2018 to October 2019 were included. Patients were classified into the good genotype group for clopidogrel metabolism (ultrarapid or extensive metabolizer) and poor genotype group (intermediate/unknown or poor metabolizer) based on their CYP2C19 genotype status. A scanning capillary-tube viscometer was used to assess whole BV, and patients were divided into decreased BV and increased BV groups.
Results
The final analyses included 174 patients (109 men and 65 women) with a mean age of 66.4±11.2 years. The good genotype was found in 44% of patients with decreased systolic BV (SBV) and 27% of those with increased SBV (P=0.029), suggesting that BV changes were related to the CYP2C19 genotype for clopidogrel metabolism. Binary logistic regression analysis showed that CYP2C19 genotype status (P=0.024) and baseline SBV (P<0.001) were significantly associated with decreased BV. The good genotype for clopidogrel metabolism was associated with decreased BV in patients with ischemic stroke treated with clopidogrel.
Conclusion
The present results indicate that the effect of clopidogrel treatment on ischemic stroke prevention could be modulated not only by inhibition of platelet function but also by changes in the hemorheological profile.
INTRODUCTION
Blood viscosity (BV) is a measurement of the intrinsic resistance of blood to flow and is characterized by blood thickness and stickiness [1-3]. High BV increases thromboembolic risk and plays an important role in cardiovascular disease [2-6]. We previously reported that prior antithrombotic use is significantly associated with decreased BV in patients with acute ischemia [7]. Our findings demonstrate that prior antithrombotic medication may change the hemorheological profile in the acute phase of ischemic stroke. Enhancement of erythrocyte deformability and inhibition of platelet aggregation may be related to decreased BV when antithrombotics are used. Evidence has begun to develop regarding the relationship between cytochrome P450 2C19 (CYP2C19) polymorphisms in clopidogrel-treated patients and stroke recurrence following ischemic stroke [8,9]. Our previous study revealed that in patients with acute ischemic stroke treated with clopidogrel, a good CYP2C19 genotype for clopidogrel metabolism was associated with a 31% decrease in the relative risk of recurrent stroke [10]. Informative studies on pharmacological therapies for reducing BV are limited [11,12]. One study showed that in patients with subclinical carotid or femoral atherosclerosis, clopidogrel reduces the mean low-shear BV by 18% after 3 weeks of treatment [12]. Aspirin with dipyridamole is also more effective than aspirin alone in reducing low-shear BV [13]. However, there is no evidence that CYP2C19 polymorphisms in clopidogrel-treated patients influence BV after ischemic stroke.
We hypothesized that CYP2C19 polymorphisms in clopidogrel treatment may inhibit platelet aggregation, which is related to BV. We presumed that a good genotype for clopidogrel metabolism may decrease BV, and this effect could be demonstrated by serial BV measurements. In this context, this study was designed to evaluate the difference between the propensities of a good genotype group for clopidogrel metabolism and a poor genotype group to reduce BV in patients with ischemic stroke on clopidogrel treatment.
METHODS
Patients
For this study, patients (aged ≥40 years) who developed ischemic stroke or transient ischemic attack (TIA) within 7 days of symptom onset from April 2018 to October 2019 were enrolled. Weakness, speech disturbance, dysarthria, or dysphasia for >5 minutes, had to be part of the symptom complex of TIA for patients to be eligible [14]. Patient demographics and clinical information were assessed at admission. An expert pharmacist checked all medications that each patient took regularly during the week that preceded their admission. Systemic investigations were performed for all patients. Each patient underwent brain magnetic resonance imaging or computed tomography (CT) and at least one vascular imaging study, such as magnetic resonance angiography or CT angiography. Echocardiography and 24-hour Holter monitoring were performed in selected patients to detect the potential cardiac sources of embolism. Stroke subtype classification was performed according to the Trial of ORG 10172 in the Acute Stroke Treatment classification system [15]. Patients with high-risk cardiac sources of emboli or stroke of other determined etiology as a stroke subtype were excluded from this study. All patients received appropriate treatment, including antihypertensive or antidiabetic drugs or statins, during the study.
BV measurement
The methods of BV measurement used in this study have been published previously [7]. A scanning capillary-tube viscometer (SCTV; Hemovister; Pharmode Inc., Seoul, Korea) was used to assess whole BV. The SCTV assesses systolic BV (SBV) and diastolic BV (DBV). SBV and DBV characterize viscosities at high and low shear rates, respectively. In this study, whole BV measured at a shear rate of 300 s−1 was selected as the SBV and at 1 s−1 as the DBV [7]. Laboratory tests, including BV, hemoglobin (Hb), hematocrit (Hct), and platelets, were conducted at admission and 180±30 days after the onset of stroke. All BV samples were obtained before hydration therapy, and measurements were taken within 24 hours after collection. For the study, patients were divided into decreased BV and increased BV groups. Decreased BV suggested that the BV value in the baseline study minus the value in the 180-day study was positive, and vice versa. If the calculated BV values were zero or discordant between SBV and DBV, they were excluded from the study (nine patients).
CYP2C19 genotyping assay
The methods used for the CYP2C19 genotyping assay have been published previously [10]. In brief, the CYP2C19 genotype of the study population was measured using the Real-Q CYP2C19 genotyping kit (Biosewoom, Seoul, Korea) and the Seeplex CYP2C19 ACE Genotyping system (Seegene, Seoul, Korea). Patients were classified as an ultrarapid metabolizer (UM; *1/*17, *17/*17), extensive metabolizer (EM; *1/*1), intermediate (IM)/unknown metabolizer (*1/*2, *1/*3 and *2/*17, *3/*17), or poor metabolizer (PM; *2/*2, *2/*3, *3/*3) based on CYP2C19 genotype status. For this study, UM or EM status patients were allocated to the good genotype group for clopidogrel metabolism, while IM/unknown metabolizer or PM status patients were allocated to the poor genotype group.
Statistical analysis
Variables were verified for normality using the Kolmogorov-Smirnov test. Descriptive data are expressed as number (percentage) or mean±standard deviation. Categorical data were examined using the chi-square or Fisher’s exact test. Univariate analyses of patient characteristics were performed using an independent-samples t-test or the Mann-Whitney U-test for continuous variables and the chi-square or Fisher’s exact test for categorical variables. Binary logistic regression analysis was performed to examine the association of univariate variables. Two-sided null hypotheses of no difference were rejected if P-values were less than 0.05. Statistical analyses were performed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Fig. 1 shows the study flow and the identified reasons for exclusion from the study. A total of 539 patients who had experienced ischemic stroke or TIA within 7 days of symptom onset were screened, of which 294 (55% of the screened population) were excluded from the study. One hundred and sixty-two patients (30%) refused to participate in the study, and 132 (25%) were excluded based on the exclusion criteria. During the study, 71 patients (13%) did not follow the study protocol, and as a result, 174 (32%) were included in the final analysis. The most frequent stroke subtype was lacunar stroke (n=82, 47%), followed by stroke of undetermined etiology, negative work-up (n=50, 29%), large artery atherosclerosis (n=28, 16%), and TIA (n=14, 8%).

Study profile. TIA, transient ischemic attack; CE, cardioembolism; SUDm, stroke of undetermined etiology (SUD), more than two causes identified; SUDi, SUD, incomplete evaluation; SOD, stroke of other determined etiology; SUDn, SUD, negative work-up; LAA, large artery atherosclerosis.
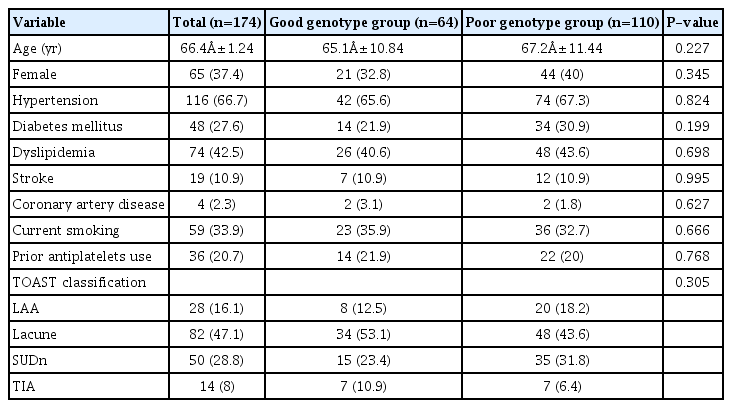
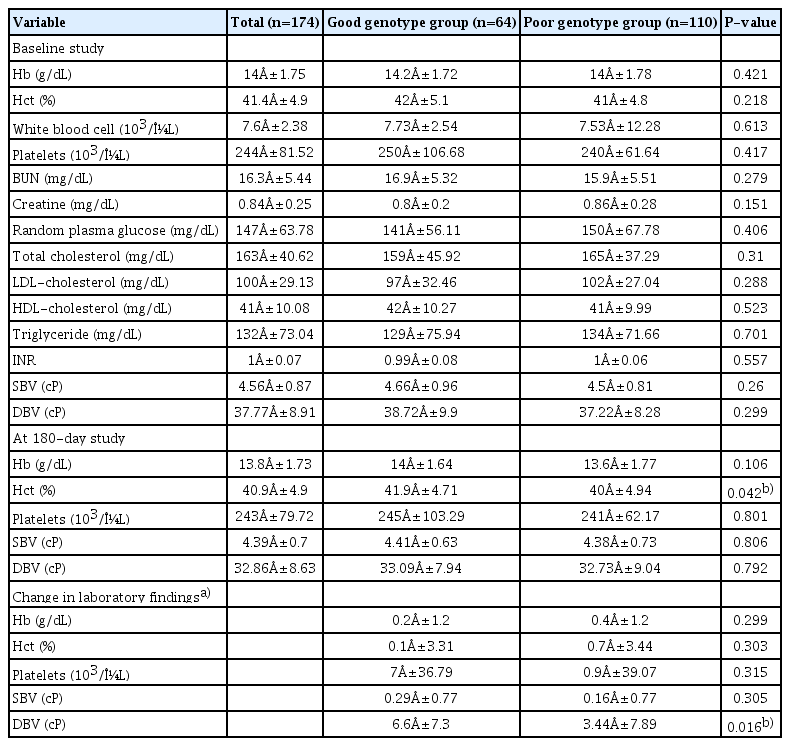
The baseline characteristics of the study population according to CYP2C19 genotype status are shown in Table 1. The mean age was 66.4±11.2 years, and 37% of the patients were women. Of these, 67% had a history of hypertension, 28% had a history of diabetes, 43% had a history of dyslipidemia, 11% had a history of stroke, and 2% had a history of coronary artery disease (CAD). In addition, 37% of the patients had a good genotype for clopidogrel metabolism, and 34% were current smokers. There were no significant differences in the baseline characteristics between the two groups. Regarding prior antiplatelet therapy, 36 patients (21%) were regularly taking antiplatelet drugs (aspirin alone, 36%; clopidogrel plus aspirin, 25%; clopidogrel alone, 20%; others, 19%) at admission. Although there were no differences in baseline BV values among the different antiplatelet therapy groups (P=0.446), a trend toward a decreased baseline BV value was observed in the prior treatment group (P=0.137 for SBV and P=0.125 for DBV). Table 2 shows the laboratory findings according to CYP2C19 genotype status. There were no differences in the baseline findings between the two groups. In the 180-day study, Hct and changes in DBV were significantly higher in the good genotype group. No differences were observed in other laboratory findings between the groups.
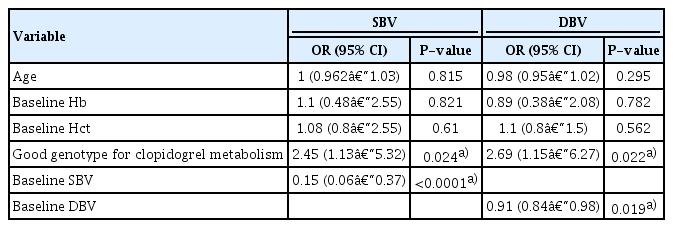
The decreased-SBV group had a better genotype for clopidogrel metabolism than the increased-SBV group on clopidogrel treatment (Table 3). The good genotype was found in 44% of the decreased-SBV patients and 27% of the increased-SBV patients (P=0.029), suggesting that BV changes were related to the CYP2C19 genotype for clopidogrel metabolism. The analysis using DBV demonstrated the same results (P=0.013). Baseline Hb, Hct, SBV, and DBV values were significantly higher in the decreased-SBV group. A trend toward older age was observed in the increased-SBV prior treatment group (P=0.053). Binary logistic regression analysis using age, baseline Hb, Hct, SBV value, and CYP2C19 genotype status revealed that CYP2C19 genotype status (odds ratio [OR], 2.45; 95% confidence interval [CI], 1.13–5.32; P=0.024) and baseline SBV (OR, 0.15; 95% CI, 0.06–0.37; P<0.001) were significantly associated with decreased SBV (Table 4). Regarding DBV, the analysis using age, baseline Hb, Hct, DBV value, and CYP2C19 genotype status revealed that CYP2C19 genotype status (OR, 2.69; 95% CI, 1.15–6.27; P=0.022) and baseline DBV (OR, 0.91; 95% CI, 0.84–0.98; P=0.019) were strongly correlated with decreased DBV.
During the study, clopidogrel plus aspirin was administered to 144 patients (83%), and clopidogrel alone was administered to the remaining 17%. The baseline characteristics and laboratory indices according to SBV changes and type of antiplatelet therapy are shown in Table 5. In the decreased-SBV group, the proportion of patients with prior stroke and those who had prior antiplatelet therapy were higher in those receiving clopidogrel alone (P=0.003), related with previous aspirin use for secondary stroke prevention. In the increased-SBV group, female sex and prior antiplatelet use were higher in the clopidogrel alone group (P=0.023). No differences were observed in the SBV and DBV changes among the groups.
DISCUSSION
In this study, we evaluated the propensity of a good genotype for clopidogrel metabolism and a poor genotype to reduce BV using serial BV measurements. Our study clearly demonstrated that a good genotype for clopidogrel metabolism was associated with decreased BV in patients with ischemic stroke treated with clopidogrel. Logistic regression analysis showed that CYP2C19 genotype status and baseline BV were related to decreased BV.
Several studies have revealed an association between BV and the occurrence of major thromboembolic events [16,17]. As for stroke, BV is elevated in acute ischemic stroke. After a discrete increase in the acute phase, a gradual improvement is observed in the chronic phase [18]. BV appears significantly higher in cases of lacunar or cardioembolic strokes [1,3,19]. BV may contribute to the onset of stroke subtypes in a different way and may be related to the pathogenesis of thrombus formation [1]. The major determinants of BV are the aggregation and deformability of red blood cells, Hct, and plasma viscosity [2]. When BV increases with changes in blood components, flow resistance may markedly increase in stenotic perforating arteries. This hemorheological change could be associated with thrombus formation in lacunar infarctions [1,7].
Although we reported that prior antithrombotic use was significantly associated with decreased BV within 24 hours of symptom onset in patients with acute ischemic stroke [7], there was only a trend toward decreased BV in the prior antiplatelet treatment group (P<0.137) in this study. BV remains uniform over a short period, and the median time from symptom onset to admission was 3.2 days in this study. One study showed that BV was significantly higher at admission but was normalized after 2 weeks of hydration [5]. Differences in BV measurement time and sample size may be plausible explanations for these discrepancies between our studies.
In our study, a good genotype for clopidogrel metabolism was positively associated with decreased-BV. BV can be modified by medical therapies, including vasodilators, statins, or antithrombotics [13,20,21]. Few studies have demonstrated the effect of antithrombotics on BV [13,21,22]. Warfarin, heparin, and argatroban decrease BV [21,23]. Warfarin reduces BV significantly in patients with acute ischemic stroke with nonvalvular atrial fibrillation, compared to aspirin [21]. Regarding antiplatelet therapy, results differed depending on the study protocol. Aspirin and cilostazol do not change BV, but dipyridamole and clopidogrel decrease BV after treatment [11,13,21,24]. Clopidogrel, the P2Y12 receptor antagonist, could increase the adenosine and cyclic adenosine monophosphate plasma concentration, which have been shown to lower BV [13,25]. It is also known that clopidogrel is associated with improvement in microvascular reactivity in patients with CAD [26]. Clopidogrel may have a positive influence on hemorheological parameters. Therefore, the effect of clopidogrel on ischemic stroke prevention could be modulated not only by inhibition of platelet function but also by changes in the hemorheological profile [11,26].
The strength of this study its longitudinal design using serial BV examinations. Unlike simple cross-sectional studies, this study was used to estimate the effect of CYP2C19 genotype and BV changes in clopidogrel-treated patients with ischemic stroke. There were several limitations in this study, including small sample size. Of the 539 patients screened during the study, only 174 (32%) were enrolled. However, our study showed that 37% of participants belonged to the good genotype group for clopidogrel metabolism. These results were in concordance with those of previous studies reporting that approximately 41% of Koreans belonged to the good genotype group [27]. Second, we could not measure plasma components such as fibrinogen or C-reactive protein in all patients. BV can change according to the levels of other aggregating proteins. Third, the response to clopidogrel might have been affected by several clinical factors, including sex and smoking [10]. We could not evaluate the clinical factors associated with variability in the response to clopidogrel. Finally, clopidogrel plus aspirin was given to 144 patients (83%). Although aspirin does not change BV [13], dual antiplatelet therapy with clopidogrel plus aspirin may affect the BV changes differently. No differences were observed in BV changes between the clopidogrel plus aspirin and clopidogrel alone treatment groups in our study. However, the small sample size (144 vs. 30) limits the generalizability of our findings. These limitations should be considered when interpreting our data.
In conclusion, a good genotype for clopidogrel metabolism was associated with decreased BV in patients with ischemic stroke after clopidogrel treatment. Our findings indicate that the effect of clopidogrel treatment on ischemic stroke prevention could be modulated not only by inhibition of platelet function but also by changes in the hemorheological profile. Further studies that focus on the CYP2C19 genotype and BV are essential to evaluate the effects of clopidogrel treatment on stroke recurrence.
Notes
Ethics statement
The Research Ethics Committee of Inje University Sanggye Paik Hospital approved the present study (IRB No. 2019-05-021). The requirement for informed consent was waived because the database was accessed only for purposes of analysis and personal information was not used.
Conflict of interest
No potential conflict of interest relevant to this article.
Author contributions
Conceptualization: SWH. Data curation, Formal analysis, Investigation, Methodology: JHP, SWH. Project administration: JHP. Resources & Software: HJY. Supervision: HJY, SWH. Validation: HJY. Visualization & Writing–original draft: JHP, SWH. Writing–review editing: SWH, HJY.