Multiple embolic infarctions and intracranial hemorrhage in a patient with gestational trophoblastic disease
Article information
Abstract
Background
Choriocarcinoma is a subtype of gestational trophoblastic disease (GTD) that can spread to multiple organs, including the central nervous system. Most cases of GTD affecting the central nervous system can cause intra- or extra-axial hemorrhages. Herein, we describe a rare case of multiple embolic infarctions and intracranial hemorrhages in a patient with GDT.
Case Report
A 36-year-old woman with sudden headache and right homonymous hemianopsia was admitted to our hospital 19 hours from symptom onset. Brain magnetic resonance imaging revealed small acute infarctions in the territories of the left posterior cerebral artery and both middle cerebral arteries. Furthermore, intracerebral hemorrhage in the left occipital lobe, small amounts of intraventricular hemorrhage, and subarachnoid hemorrhage were observed. In the past, she gave birth to her child through cesarean section 6 months ago. D-dimer level was elevated with a value of 1.61 µg/mL (reference range <0.5 µg/mL). Her urine beta-human chorionic gonadotropin (hCG) was positive, and her serum beta-hCG level was >1,000 IU/mL. She was diagnosed with GTD and underwent chemotherapy.
Conclusion
The precise pathogenesis of the coexistence of multiple embolic infarctions and intracranial hemorrhage remains unclear. Cancer-related coagulopathy, micro-tumor emboli, or both could be involved in the pathogenesis of the rare presentation of this patient.
INTRODUCTION
Gestational trophoblastic disease (GTD) is a clinical spectrum consisting of benign hydatidiform mole, invasive mole, and highly malignant choriocarcinoma. Choriocarcinoma can spread rapidly through the bloodstream to the lungs, vagina, liver, kidney, ovaries, and brain. Approximately 20% of patients with choriocarcinoma have central nervous system (CNS) involvement, with diverse symptoms [1]. Most cases affecting the CNS include intra- or extra-axial hemorrhages due to oncotic aneurysm formation and its subsequent rupture [2]. Herein, we report a case of a patient with GTD with rare multiple embolic infarctions and intracranial hemorrhage.
CASE REPORT
A 36-year-old woman with sudden headache and right homonymous hemianopsia that occurred 19 hours before presentation was admitted to the emergency room (ER) on November 25, 2016. She had experienced facial palsy in the left side and clumsiness in the left arm 3 months before presentation, but spontaneously recovered within a week. One month later, she experienced weakness and numbness in her left hand. She experienced intermittent headaches intermittently for 15 days. The headache worsened and was accompanied by nausea and vomiting from the day before the ER visit.
In the past, she gave birth to her child through cesarean section 6 months ago. She had no family history of stroke. Brain magnetic resonance imaging (MRI) revealed small acute infarctions in multiple vascular territories, including the left posterior cerebral artery and both middle cerebral arteries (MCAs). Furthermore, intracerebral hemorrhage (ICH) in the left occipital lobe, small amounts of intraventricular hemorrhage (IVH), and subarachnoid hemorrhage (SAH) were observed. There was no significant vascular derangement on her magnetic resonance angiography (Fig. 1). Initial blood pressure and body temperature were 188/89 mmHg and 36.8°C, respectively. We performed an emergency transthoracic echocardiography to assess infective endocarditis. There was no evidence of vegetation or any embolic source, such as patent foramen ovale, ischemic heart disease, or valvular disease. Normal sinus rhythm was observed on electrocardiography. Complete blood counts, erythrocyte sedimentation rate, urinalysis, plasma electrolyte, kidney, liver, and thyroid function tests were normal. D-dimer level was elevated with a value of 1.61 µg/mL (reference range <0.5 µg/mL). We performed chest and abdominal computed tomography (CT) to rule out malignancy. Chest CT revealed clustered nodular and tubular opacities, and tree-in-bud appearances were observed in both lungs, which were indicative of pulmonary tumor thrombotic microangiopathy (PTTM) (Fig. 2). There were no abnormal findings on her abdominal CT. Her urine beta-human chorionic gonadotropin (hCG) was positive, and serum beta-hCG level was >1,000 IU/mL. The elevated level of beta-hCG in her serum and the presence of PTTM in a patient of reproductive age strongly suggested GTD. However, the pathology result of the endometrial biopsy was nonspecific.

Initial brain magnetic resonance imaging shows multiple embolic infarctions in the multiple vascular territories (A-E). Intracerebral hemorrhage in the left occipital lobe and adjacent intraventricular, subdural and subarachnoid hemorrhages are also observed on susceptibility-weighted images (F-I). No significant vascular derangement is seen on the magnetic resonance angiography (J).
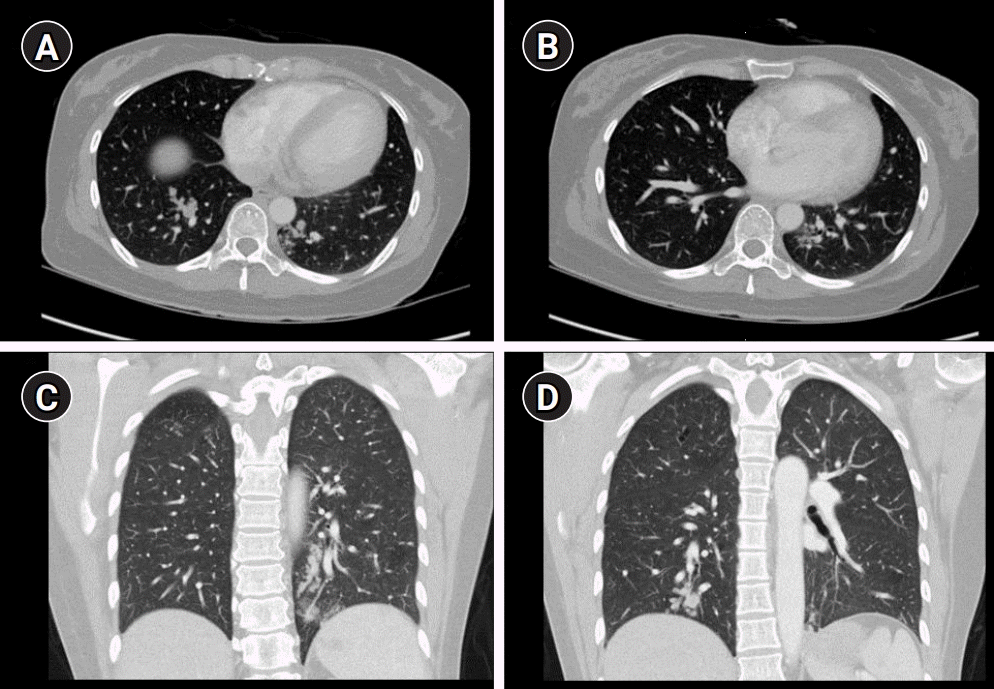
Axial (A, B) and coronal (C, D) views of the chest computed tomography show clustered nodular and tubular opacities, as well as tree-in-bud appearances in both the lungs.
With intravenous nicardipine and labetalol administration, her systolic blood pressure was lowered immediately to 140 mmHg or less to prevent hematoma expansion. She was administered a dose of mannitol (40 g; 2 bottles of 20% mannitol; 100 mL) four times a day for 5 days and then tapered off over 4 days. Combinations of nonsteroidal anti-inflammatory drugs and opioids have also been used to control headache. Her follow-up CT confirmed that there was no hemorrhage expansion and rebleeding. After mannitol tapering, she was transferred to the department of oncology. At that time, she complained of a mild headache and right homonymous hemianopsia, and there was no other neurological deficit on her neurological examination. She started chemotherapy with etoposide, methotrexate, actinomycin-D, cyclophosphamide, and vincristine on December 5, 2016. The level of beta-hCG decreased dramatically to the normal range (8.0 IU/mL on January 16, 2017). Cerebrospinal fluid (CSF) analysis was performed on December 19, 2016. The white blood cell and red blood cell counts in the CSF were 0 /mm3 and 3 /mm3, respectively. The CSF protein level was in the normal range (32.2 mg/dL). There was no evidence of malignancy on CSF cytology. The patients completed chemotherapy on November 7, 2017. Follow-up brain MRIs on November 14, 2016, January 1, 2017, and February 27, 2017, revealed no recurrent stroke. Brain MRI has been performed every year to check for recurrent brain lesions. Lately, she underwent brain MRI on July 24, 2020, and there was no evidence of recurrence. To date, there have been no neurological deficits other than visual field defects.
DISCUSSION
Choriocarcinoma is a very aggressive, malignant variant of GTD, which grows rapidly and metastasizes to the lung, liver, and, less frequently, to the brain. The metastatic involvement of the lung in our case suggested that the clinical pathologic form of this patient was choriocarcinoma. Most cases of choriocarcinoma involving the CNS present with both intra- and extra-axial hemorrhage. These were manifested by vascular fragility and rupture of oncotic aneurysms. Arterial infarctions due to direct tumor emboli are also possible, but there was no evidence of vascular tumor invasion on serial contrast-enhanced MRI [2]. If the ratio of serum to CSF hCG is less than 60, CNS metastasis is strongly suspected. Unfortunately, we performed CSF analysis two weeks after chemotherapy because increased intracranial pressure was assumed during the period of acute ICH, IVH, and SAH. The ratio of serum to CSF hCG could not be obtained [3]. Acute cerebral infarctions in multiple vascular territories and elevated level of D-dimer (1.61 µg/mL) were observed in this case. These findings suggest that the main cause of multiple cerebral infarctions and hemorrhage could be cancer-associated hypercoagulability rather than direct tumor emboli. A previous study showed that concealed cancer should be considered in patients with ischemic lesions in multiple vascular territories on diffusion-weighted imaging (DWI). The level of D-dimer, a specific derivative of cross-linked fibrin, has been used in many previous studies as a measure of hypercoagulability [4]. The DWI pattern of ischemic lesions in multiple vascular territories and elevated D-dimer level (>1.11 µg/mL) was independently associated with cancer-related coagulopathy (CRC) [5,6]. In these scenarios, ICH could occur in the preexisting infarct area. There have been a few case reports of metastatic choriocarcinoma as a cause of ischemic and hemorrhagic stroke [7-9]. The case reported by Bonnet et al. [7] was very similar to our case in terms of the concomitant presence of multiple ischemic stroke and lobar hematoma. In this case, a local thrombus in the left atrium was detected on transesophageal echocardiography (TEE). It could be considered that thrombotic embolus was the most plausible diagnosis. However, there was no evidence of hypercoagulability in her laboratory findings [7]. Another case had multiple distributed, small, and large infarctions. Her neurological state deteriorated 3 days later. Hemorrhagic transformation of the large infarction in the left MCA territory was observed [9]. The precise pathogenesis of multiple small and large infarctions remained uncertain. In our case, D-dimer levels were elevated, suggesting hypercoagulability. Although we could not perform TEE, there was no evidence of non-bacterial thrombotic endocarditis on TTE. Several cases of choriocarcinoma with pulmonary thromboembolism have been reported previously [10]. The effects of thrombolytic therapy, embolectomy, and/or anticoagulant therapy were uncertain in these cases. The literature highlights the importance of early diagnosis and timely appropriate chemotherapy in the management of this condition [10]. In our case, the patient was diagnosed with PTTM. The pathomechanism of PTTM involves tumor cells entering the pulmonary circulation; this occludes small arterioles and expresses vascular endothelial growth factor and tissue factor that lead to proliferation of intimal myofibroblasts and luminal stenosis. The coexistence of the PTTM and stroke suggested that the pathomechanisms of these were similar [11]. The arterial tumor emboli could have occurred when the metastatic pulmonary neoplasm invaded the heart through the pulmonary vein. Although there was no evidence of brain metastasis on the serial contrast-enhanced MRI and CSF analysis, there were possibilities of micro-thromboembolism, micrometastasis, or micro-tumor embolism, which cannot be detected on MRI. Choriocarcinoma may metastasize to the cerebral blood vessels, resulting in ischemic stroke or intraparenchymal hemorrhage [8,12]. Furthermore, the coexistence of micro-tumor embolism with CRC could not be excluded in our case. The ratio of serum to CSF hCG before chemotherapy may be important to confirm CNS metastasis. However, we checked the CSF after the initiation of chemotherapy because of the presence of increased intracranial pressure. Timely appropriate chemotherapy was very important in this condition.
This is a rare case of multiple embolic infarctions and intracranial hemorrhages in a patient with GTD. The precise pathogenesis of coexisting ischemic and hemorrhagic strokes is not clear. CRC, micro-tumor emboli, or both could be the pathogenesis of this rare presentation.
Notes
Ethics statement
This case was reviewed and approved by the Institutional Review Board of Gyeongsang National University Changwon Hospital (IRB No. 2020-12-032). Informed consent was waived by the Board.
Conflict of interest
No potential conflict of interest relevant to this article.
Author contributions
Conceptualization: KYK, SJ, CY, KJP. Visualization & Writing–original draft: KYK, SJ. Writing–review & editing: HJ, EBC, TWY, SJK, SH.
