A Case of Fentanyl Intoxication and Delayed Hypoxic Leukoencephalopathy Caused by Incidental Use of Fentanyl Patch in a Healthy Elderly Man
Article information
Abstract
Background:
Fentanyl intoxication has been reported occasionally since the Fentanyl patch became available. Delayed hypoxic leukoencephalopathy is recognized as a complication of hypoxic events. However, its neuropsychiatric symptoms can be delayed.
Case Report:
An 85-year-old male presented with mental deterioration after attaching a Fentanyl patch. A hypoxic condition was detected. Neurological examination revealed semi-coma, pupil myosis, sluggish light reflex, and no response to deep pain. He recovered gradually but showed delayed neuropsychiatric symptoms 40 days after the hypoxic event. These symptoms were improved steadily. He could talk and walk after 3 months.
Conclusion:
The case of acute fentanyl intoxication and delayed hypoxic leukoencephalopathy reported here is caused by incidental use of a Fentanyl patch in a healthy elderly man. When patients are admitted with mental deterioration, respiratory depression, and pupil miosis, physicians should consider opioids intoxication including Fentanyl patch. In addition, it is important to understand the clinical course of leukoencephalopathy as a delayed complication after recovery from acute fentanyl intoxication.
INTRODUCTION
Fentanyl is a selective µ-receptor agonist and a synthetic narcotic analgesic with strong analgesic effect. Due to its rapid action and a short duration of action in the body, it has been used to control acute pain [1]. The concentration of a Fentanyl patch in the body generally levels off after 12–24 hours and remains relatively constant for up to 3 days through transdermal passive diffusion after adherence to the skin [2]. Because of its phamacokinetic properties, the transdermal fentanyl patch should be used to control chronic pain in patients who do not respond to less potent analgesic drugs. However, Fentanyl patch has many side-effects, such as deterioration of the central nervous system, respiratory depression, hypothermia, cold wet skin, flaccid muscles, bradycardia, and hypotension [3]. Fetal fentanyl intoxication has been reported occasionally since the fentanyl patch became available, because its noninvasiveness and simple method of administration result in abuse for managing acute pain, such as muscle pains and strains. Also lethal side-effects, such as respiratory depression and hypotension, have been sometimes reported with use of the fentanyl patch for controlling postoperative pain [4].
Delayed hypoxic leukoencephalopathy is a rare disease caused by carbon monoxide poisoning, inhaling toxic gas, cardiac arrest, and overdose of opiates or benzodiazepines [5,6]. Symptoms of delayed hypoxic leukoencephalopathy, such as cognitive impairment, gait disorder, parkinsonism, and akinetic mutism, appear at different times from a few days to weeks after the patient regains consciousness from hypoxia [7].
We report a case of delayed hypoxic leukoencephalopathy that occurred after fentanyl intoxication caused by incidental fentanyl patch use in a healthy elderly man.
CASE REPORT
An 85-year-old male patient was transferred to the emergency room due to reduced consciousness by ambulance. Rescue men ventilated the patient artficially with the bag-valve-mask connecting a line with 100% concentration of oxygen because oxygen saturation level is 55% and pulse rate is 110/min in ambulance. His vital signs at admission were: blood pressure, 60/40 mmHg; respiratory rate, 9/min; pulse rate, 112/min; and body temperature, 37.8°C. An arterial blood gas analysis (ABGA) revealed pH, 7.29; PaCO2, 60 mmHg; PaO2, 77 mmHg (normal range, 83–108 mmHg); HCO3−, 28.9 mmol/L; base excess, 0.9 mmol/L; and blood oxygen saturation level, 90%. The presentations of cyanosis were not discovered but bradypnea and shallow respiratory movements were seen during a physical examination of the chest, so intravascular vasopressors, intubation, and mechanical ventilation were applied as soon as possible. The vital signs recovered (blood pressure, 120/70 mmHg; respiratory rate, 12/min; pulse rate, 110/min; and body temperature, 37.5°C), and all ABGA levels were normal. He had a semi-comatose mentality, pupil miosis, a sluggish pupillary light reflex, and no response to deep pain in any limb on a neurological examination. No nuchal rigidity, abnormal deep tendon reflex, or any other lateralizing signs were detected. A complete blood count showed WBC, 22,400/uL; neutrophils, 91% and others within the normal range. A routine chemistry analysis revealed Na, 130 mmol/L; K, 4.6 mmol/L; Cl, 94 mmol/L; BUN, 20.1 mg/dL; Cr, 1.49 mg/dL; T-protein, 5.933 g/dL; albumin, 3.605 g/dL; CRP, 0.881 mg/dL; glucose, 168 mg/dL; s-Osm, 289 mosm/kg; CK, 128 U/L; ammonia, 48 µg/dL; and lactate, 0.6 mmol/L. A cardiac marker showed CK-MB, 3.5 ng/mL; and troponin-I 0.231 pg/mL. The next day several laboratory levels were increased (CK, 280 U/L; CK-MB, 6.7 ng/mL; and troponin-I 0.526 pg/mL). In addition, no abnormal findings were seen on a brain computed tomography (CT) and chest X-ray conducted in the emergency room.
No specific events were found in his medical history, and the patient had suffered no particular problem until he went to sleep the night before admission. We found a Fentanyl patch attached on his right wrist and got his history that he attached the patch prescribed to his friend who had cancer pain on a painful right wrist and went to bed the day before admission. The next day morning he was found unconscious. After conservative treatment, such as mechanical ventilation and hydration with normal saline mixed with naloxone in the intensive care unit (ICU), he regained consciousness. No abnormalities were detected on an electroencephalogram (EEG) or brain magnetic resonance imaging (MRI) (Fig. 1A). The patient was transferred to Respiratory Medicine because of aggravated aspiration pneumonia that had occurred on admission. He had exhibited abnormal behaviors, such as shouting, disconnecting phone lines, and urinating on the bed in the last 20 days since the hypoxic event, and was discharged from respiratory medicine.
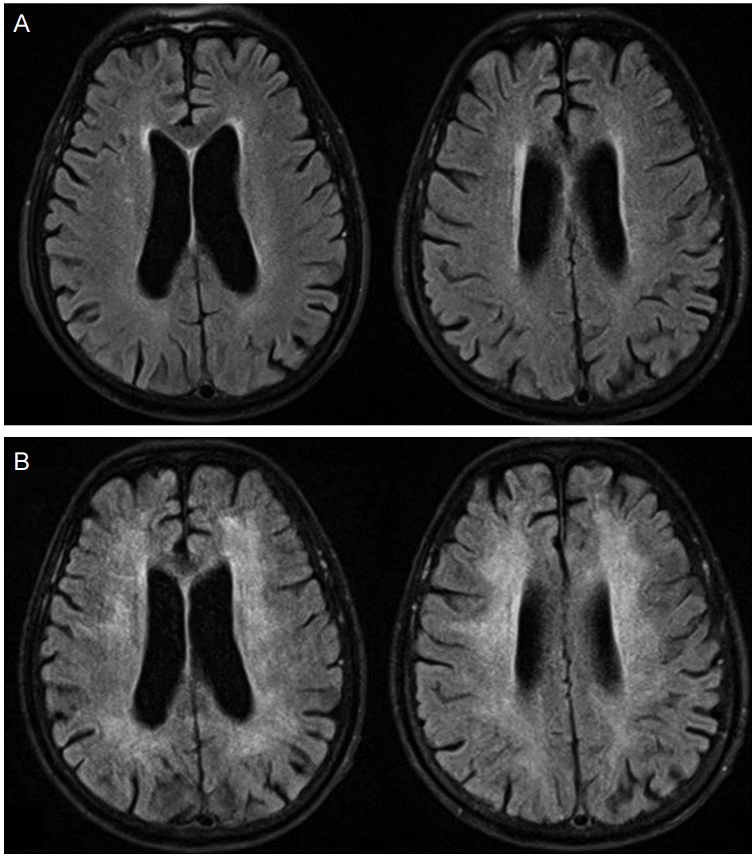
Brain magnetic resonance images. Fluid attenuated inversion recovery (FLAIR) images of the patient on admission day (A) and following FLAIR images 40 days after the exposure of a fentanyl patch (B) show bilaterally symmetrical high signal intensities on deep and periventricular white matter without involvement of gray matter, which were not observed in previous study.
The patient was readmitted to the neurology ward 20 days after discharge (40 days since the hypoxic event) due to poor activities of daily living, such as impaired ambulation, mutism, not using the toilet, and overall memory loss including short- and long-term memory. The neurological examination revealed alert mentality without awareness and akinetic mutism but no other focal neurological abnormalities were observed. Brain MRI taken 45 days after the hypoxic event showed bilaterally symmetrical high signal intensity in the white matter on a fluid attenuated inversion recovery (FLAIR) image (Fig. 1B). After diagnosing delayed hypoxic leukoencephalopathy induced by the Fentanyl patch, walking and speaking improved through conservative and rehabilitative therapies from early bedside physical training to simple gait. Intravenous methylprednisolone was started as a possible anti-inflammatory and neuro-protective drug at a dose of 1 g daily for five days and oral methylprednisolone was taken and tapered next five days. Also neurotonics such as choline alfoscerate and donepezil were administrated consistently. After 1 month, he was discharged and went to a neighboring geriatric hospital.
The patient did not use the toilet 3 months after discharge and developed a sleep disorder and aggressiveness. He died of aspiration pneumonia in the geriatric hospital 5 months after the second discharge.
DISCUSSION
In our case, intoxicate symptoms and signs including hypoxia occurred after a Fentanyl patch was attached incidently, and the neurological deficits of gait impairment, akinetic mutism, and memory loss were detected after 40 days since the hypoxic event. Delayed hypoxic leukoencephalopathy was diagnosed on a brain MRI scan 45 days after the its intoxication and hypoxic event.
Respiratory depression with deterioration of the central nervous system was caused more in patients using opiates for the first time than in the long term to control chronic pain because of the tolerance to opioid drugs [8]. In our case the patient had no history about past fentanyl exposures.
The reason that symptoms of delayed hypoxic leukoencephalopathy can be confusing is clinicians cannot quickly conclude these symptoms to be effects of delayed hypoxic leukoencephalopathy or other new neuropsychological diseases because the time interval between hypoxia and the delayed neurological symptoms can be a few days to weeks. Brain MRI is most helpful to diagnose delayed hypoxic leukoencephalopathy [9]. Sometimes the arylsulfatase enzyme, which is related to metachromatic leukodystrophy, decreased in patients with delayed hypoxic leukoencephalopathy, so measuring serum arylsulfatase can be helpful for the diagnosis [6].
The mechanism of delayed hypoxic leukoencephalopathy is that the activity of the myelin-producing ATP-dependent enzymatic pathway that forms cerebral white matter is inhibited by hypoxia and delayed demyelination is caused [6]. No particular treatment is known, except rehabilitation and conservative treatment but akinetic mutism in a patient with delayed hypoxic leukoencephalopathy improves rapidly after magnesium sulfate is administered intravenously [10]. The neurological sequelae in our cases were relatively mild compared with reported cases of carbon monoxide intoxication. The neuroprotective effect of fentanyl may have contributed to the better prognosis [11].
In conclusion, we report a case of acute fentanyl intoxication and delayed hypoxic leukoencephalopathy caused by incidental use of a Fentanyl patch in a healthy elderly man. This case suggests that physicians working at emergency department of a hospital should consider opioid intoxication including fentanyl patch through history taking and physical examinations, when a patient is admitted with chief complaints of mental deterioration, respiratory depression, and pupil miosis. If the fentanyl patch is on the patient’s skin, it must be removed as soon as possible. Rapidly administered conservative treatments, such as artificial ventilation and naloxone injections, should provide a good prognosis. In addition, it is very important to understand clinical course of delayed hypoxic leukoencephalopathy as possible delayed complications of the patient recovered from acute opioid intoxication. If the patient is discharged only after treatments of opioid intoxication, clinicians should warn a patient and his family of capability of delayed complications. And if neuropsychological symptoms considered as delayed hypoxic leukoencephalopathy are showed, adequate imaging studies such as brain MRI and detailed history taking including opioid exposures should be performed. Through these investigations, delayed hypoxic leukoencephalopathy should be diagnosed or differentiated.