Quantitative assessments of pupillary light reflexes in neurocritically ill patients
Article information
Abstract
The pupillary light reflex is a component of bedside neurological examinations in neurological intensive care units (neuroICUs). A quantitative pupillometer provides a non-invasive and objective pupil reactivity parameter clinically significant for changes in the pupillary light reflex in neuroICUs. This article reviews the physiology and importance of the pupillary light reflex and the parameters of quantitative pupillometers. Moreover, this review discusses the clinical applications of quantitative pupillometers for post-cardiac arrest prognostication and monitoring of elevated intracranial pressure and neurological worsening in stroke, seizures, and other neurological diseases in the neuroICU. Quantitative pupillometry is a routine part of neurological examinations and an important monitoring tool for neurological diseases in neuroICUs.
INTRODUCTION
Intensive neurological examination is the most relevant diagnostic evaluation of neurological status at the bedside in patients with brain injury due to multiple etiologies and conditions predisposing them to neurological deterioration in the neurological intensive care unit (neuroICU). Monitoring of the pupillary light reflex (PLR) is a standard examination for detecting changes in cerebral dysfunction in patients with known brain injuries [1-4]. Quantitative pupillometry is an important noninvasive monitoring method of PLR that provides an objective measure of pupil diameter and pupillary reactivity [2-5]. Therefore, quantitative pupillometer monitoring has become a fundamental element of neurological assessment to quantify and standardize this aspect of neurological evaluation in neurological care. This article aims to review a quantitative pupillometer's clinical applications and impacts on neurological diseases in the neuroICU.
IMPORTANCE OF PUPILLARY LIGHT REFLEX MONITORING IN THE NEUROLOGICAL INTENSIVE CARE UNITS
Neuromonitoring of the PLR is important to detect changes in brain lesions, identify newly developed pathologies, and prevent impending secondary brain injury. In addition, changes in PLR could strongly predict neurological worsening after acute brain injury [2,3,6,7]. Under normal conditions of pupillary reactive responses, the stimulant signal of retinal cells is carried through the synapse between the optic nerve and the optic tract in the pretectum of the midbrain, which projects to the Edinger-Westphal nucleus in the dorsal midbrain [2,4,8,9]. After stimulation of the Edinger-Westphal nucleus, the parasympathetic signal is delivered by the oculomotor nerve to the superior orbital fissure of the eye and ciliary ganglion. The parasympathetic pupillary efferent signal stimulates the short posterior ciliary nerves that innervate the iris for pupil constriction [2,4,8,9]. Parasympathetic input for pupil constriction is balanced and controlled by sympathetic signals from the superior cervical ganglion for pupil dilatation. When the afferent and efferent pathways for pupil reactivity are intact in functional and structural processes, both pupils show equal sizes and brisk constriction responses when stimulated with bright light [2,4,8,9].
In neurocritically ill patients, changes and differences in both PLRs often provide early information about aggravated intracranial problems, such as increased intracranial pressure (ICP) and critical pathologies, including brainstem lesions and transtentorial herniation [10-12]. In particular, the sudden onset of a unilaterally dilated pupil, a neurological emergency condition, has been attributed to compression of the oculomotor nerve or horizontal displacement of the midbrain by increasing mass effects, leading to transtentorial herniation [10-12]. Additionally, damage to the Edinger-Westphal nuclei in the midbrain caused by ischemia, hemorrhage, or demyelinating diseases can induce abnormal PLRs bilaterally [10-12].
When performing manual pupil examinations, we usually describe the clinical features of pupil diameter and PLRs based on subjective terms as follows: unilateral (anisocoria), bilateral (isocoria), fixed, dilated, brisk, sluggish, and nonreactive [2-6]. Moreover, manual examination of the PLR has a limited inter-rater reliability of approximately 60%, as the examiners have different skills based on training levels and are allowed to use various non-standardized penlights and other light sources [13-15]. Therefore, there is a potential for compound inaccuracies and discrepancies in PLR for evaluating neurological dysfunction using manual pupil examinations [2-6,13,15].
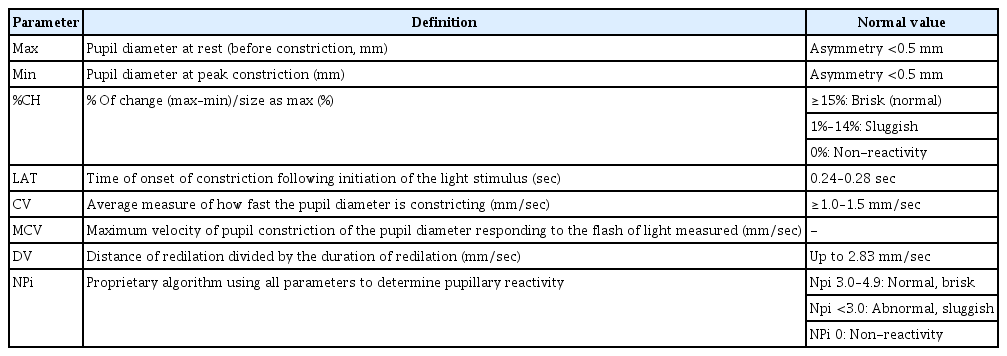
ADVANTAGES OF A QUANTITATIVE PUPILLOMETER FOR MONITORING THE PUPILLARY LIGHT REFLEX
A quantitative pupillometer is a noninvasive portable device with a light-emitting diode light source, liquid crystal display screen, and digital video camera based on infrared that measures and analyzes both PLRs. The dynamics of PLR measured by a quantitative pupillometer consisted of four phases, which were based on changes in pupil diameter over time by light stimuli (Fig. 1) [16]. A quantitative pupillometer, especially Neuroptics neurological pupil index (NPi), which measures constriction response to light, provides measured objective parameters during four phases: pupil diameter, latency, constriction velocity (CV), and dilation velocity (DV) (Fig. 2, Table 1) [4,8,17,18]. Latency (ms) is the delay time in pupil constriction following the light stimulus, which is affected by the intensity of the light and the iris smooth muscle. After the latency period, the pupil starts constricting, and the CV is analyzed and reported using changes in the pupil diameter (mm) per second (mm/sec) as well as the maximum CV (MCV). MCV is usually observed during the initial constriction phase before reaching the minimum pupil diameter. Following peak constriction, the pupil quickly starts dilating from its constricted state to its initial size. During these phases, DV was calculated using the changes in diameter over time. In addition, the percentage change (%CH) in pupil constriction was analyzed using the difference between the maximum and minimum pupil diameters. The NPi is calculated by a proprietary algorithm using multiple parameters, including latency, CVs, pupil diameter at baseline, percentage of change, and DVs (Table 1) [2-15,17,18]. In %CH, %CH ≥15% is considered a normal and brisk response, %CH <15% is a sluggish response, and 0%CH indicates a fixed pupil response. NPis of 3–5 are considered normal, NPi <3.0 is considered abnormal, and an NPi of 0 is considered a fixed pupil response (Table 1) [2-4,6-8,13,17-19]. Another quantitative pupillometer, NeuroLight-Algiscan, which measures the dilatation response of the pupil to pain, provides the PLR to gradually increase pain stimuli (Fig. 2) [4,20-22]. After baseline measurement of PLR, both PLR were assessed with electrical stimulation with gradual stepwise increasing intensity from 10 to a maximum of 60 mA applied on the left forearm connecting two electrodes to the pupillometer, with pupil diameter (mm), pupillary reflex dilation (PRD) to pain (%), and the pupillary pain index (PPI). These parameters were measured based on pupil dilation in response to increasing electrical stimulation from 10 to 60 mA with incremental steps of 10 mA for 1 second and a pulse width of 200 μs [22-24]. PPI was calculated after stopping electrical stimulation when the PRD was over 13% during stimulation. PPI of 1, the PRD is below 5% during maximal stimulation intensity (60 mA), and a PPI of 9 indicates that PRD is above 13% during the stimulation of 10 mA. In addition, a PPI score <4 is usually considered adequate for pain control [22-25]. Quantitative pupillometers provide more reliable, accurate, and objective measurements and have higher inter-rater reliability than manual pupil examinations [9,13-15,17,18].

Phases of the pupillary light reflex measured by a quantitative pupillometer. Latency is the time of onset of constriction following a light stimulus. The constriction velocity and maximum constriction velocity were calculated using the slope and maximal slope, respectively, during the constriction phase. During the dilation phase, dilation velocity was evaluated using the slope of the dilation phase. Max, maximum; Avg, average. Reprodued from Packiasabapathy et al. Can J Anaesth 2021;68:566-78 with permission of Springer Nature [16].
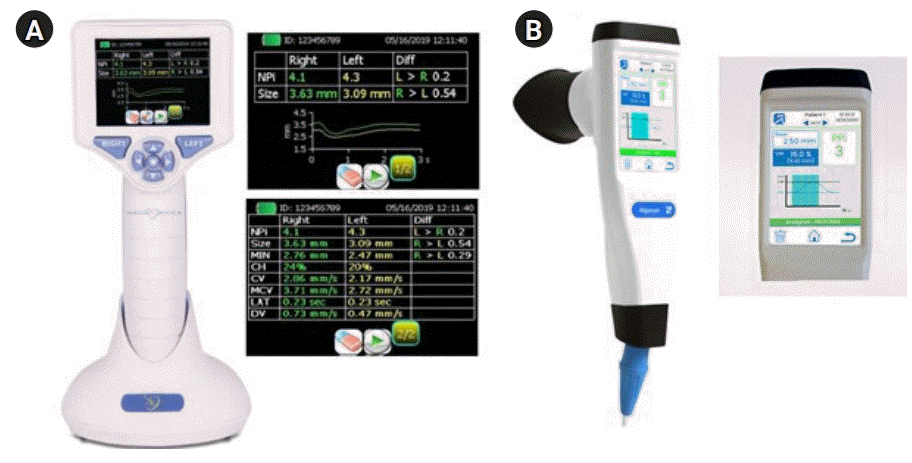
Quantitative pupillometers devices and parameters. (A) Neuroptics NPiTM-200 and result screens. (B) NeuroLight-Algiscan and result screen. Reprodued from NeuroOptics (https://neuroptics.com/npi-200-pupillometer) and IDMED (https://www.idmed.fr/en/analgesia) with permission.
CLINICAL APPLICATIONS OF QUANTITATIVE PUPILLOMETER IN NEUROLOGICAL INTENSIVE CARE UNITS
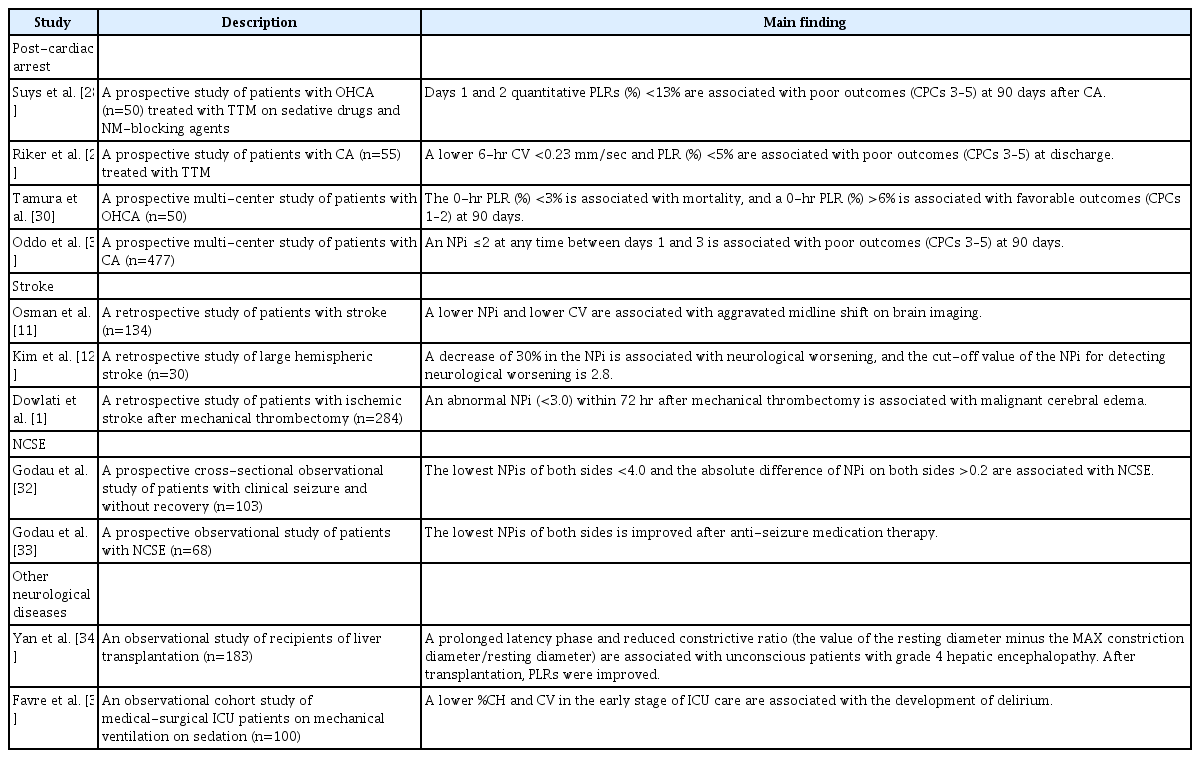
Prognosis in patients with post-cardiac arrest
Assessment of PLR has been recognized as an essential part of neurological examinations for prognostication performed after hypoxic-ischemic brain injury following cardiac arrest (CA). In particular, evaluating PLR ≥72 hours after the return of spontaneous circulation (ROSC) using a quantitative pupillometer is emerging as a helpful examination to evaluate brainstem function in post-CA patients [26,27]. Several studies have evaluated the relationship between pupillometer parameters and prognostication following CA (Table 2) [1,11,12,28-35]. A prospective study of out-of-hospital cardiac arrest (OHCA) patients treated with targeted temperature management (TTM) at 33°C with sedation and neuromuscular blocking agents reported that the quantitative %CH on days 1 and 2 were associated with poor outcomes at 90 days (cerebral performance categories [CPCs] 3–5). The day-1 quantitative %CH <13% had a sensitivity of 66.7% and a specificity of 91.3% for predicting 90-day poor neurological outcome, and the day-2 quantitative %CH <13% had a sensitivity of 63% and a specificity of 100% for predicting poor outcome at 90 days [28]. Moreover, a prospective study of 55 patients with CA treated with TTM reported that early PLR values, such as the 6-hour NPi after ROSC, predicted poor outcomes at discharge (CPCs 3–5) with an area under the curve (AUC) of 0.72 (cut-off value, 3.7) with a specificity of 82%, sensitivity of 60%, and false positive rate of 0.17. Additionally, patients with poor outcomes had lower 6-hour CVs (median value: poor outcome, 0.36 mm/sec vs. good outcome, 0.65 mm/sec) and %PLRs (%CHs) (median value: poor outcome, 8% vs. good outcome, 14%) [29]. In a prospective multicenter study of 50 patients with OHCA, 0-hour %CH ≥3% predicted 90-day survival (AUC=0.82, sensitivity=0.87, specificity=0.80) and 0-hour %CH ≥6% predicted favorable outcomes (CPCs 1–2) (AUC=0.84, sensitivity=0.92, specificity=0.74) at 90 days after CA [30]. In a prospective multicenter study of patients with CA (n=477), the predictive value of NPi for 3-month outcomes after CA was evaluated. The authors showed that an NPi ≤2 at any time between days 1 and 3 predicted poor outcomes (CPCs 3–5) 3 months after CA with 100% specificity, 32% sensitivity, 51% negative predictive value (NPV), and 100% positive predictive value (PPV). Moreover, an NPi ≤2 with bilaterally absent somatosensory evoked potentials had a higher predictive power for poor outcomes at three months after CA than an NPi ≤2 [31]. NPi also had good prognostic performance in 100 patients who received venoarterial extracorporeal membrane oxygenation therapy after refractory cardiogenic shock or CA. An abnormal NPi (<3) between 24 and 72 hours showed a predictive value of 100% specificity, 53% sensitivity, 100% PPV, and 61% NPV for 90-day mortality [36]. Regarding PPI as a prognostic value, PPI=1 at day 2 after CA predicted poor outcomes with a specificity of 100% and sensitivity of 26% in a single-center retrospective study [37]. Therefore, lower PLR parameters such as NPi, CV, and %CH were associated with poor outcomes after CA, with good predictive performance in predicting poor outcomes in patients with CA.
Monitoring in patients with increased ICP and stroke
Changes in PLR are often recognized as signs of neurological worsening due to secondary brain injury, cerebral edema, and increased ICP related to hemispheric stroke. Serial monitoring of PLRs using a quantitative pupillometer is important for the early detection of aggravation of stroke lesions and the decision of therapy to control cerebral edema and increased ICP (Table 2). In a retrospective study of 134 patients with stroke (ischemic stroke and intracerebral hemorrhage), there was a significant negative correlation between midline shift measured by the septum pellucidum and NPi, CV, and pupil asymmetry. A lower NPi is related to an aggravated midline shift on brain computed tomography or magnetic resonance imaging [11]. A study of large hemispheric stroke (n=30) reported that a sudden decrease in the NPi value, approximately 30% of the baseline NPi value, was a surrogate marker of neurological worsening and the cut-off value of the NPi for detecting neurological deterioration was 2.8 after stroke [38]. In addition, abnormal NPi values (<3.0) on the ipsilateral side of stroke lesions within 72 hours after mechanical thrombectomy had a strong independent association with malignant cerebral edema in patients with large vessel occlusion (odds ratio, 21.80; 95% confidence interval [CI], 3.32–286.4) [1]. Regarding the role of the pupillometer in increased ICP with brain herniation syndrome, several studies have shown that lower NPi values <3.0 were associated with increased ICP (mean ICP: normal NPi group, 19.6 mmHg; abnormal NPi group, 30.5 mmHg) [36] and that lower NPi values (<1.6) were associated with brain herniation syndrome with a specificity of 91% and sensitivity of 49% [39]. Moreover, repeated quantitative pupillary measurements could be a useful monitoring method of treatment in addition to early diagnostic tools for neurological deterioration in patients with acute brain injury. A study of critically ill neurologic patients with stroke or brain tumors showed that pupillary parameters such as the NPi and %CH were improved after the administration of osmotic therapy such as mannitol and hypertonic, which demonstrated the possibility of using a quantitative pupillometer as a useful monitoring method for the treatment effect in reducing brain edema [40].
Monitoring in nonconvulsive status epilepticus
Few studies have evaluated the usefulness of quantitative pupillometers for seizures (Table 2). A prospective cross-sectional observational study of 103 patients with clinical seizures without recovery to prior function examined the ability of a quantitative pupillometer to diagnose nonconvulsive status epilepticus (NCSE). The diagnosis of NCSE was established based on clinical features and electroencephalography (EEG) results, and a pupillometer was used before EEG. The NCSE groups (possible NCSE and confirmed NCSE) had the lowest NPi values on both sides (minNPi), with an optimal cut-off value of 4.0 (AUC, 0.93; 95% CI, 0.86–0.99) and a higher absolute difference of both sides (diffNPi) of 0.2 (AUC, 0.89; 95% CI, 0.80–0.99) [32]. In a prospective observational study of 68 patients with NCSE, the authors examined the changes in minNPi and diffNPi according to treatment response during anti-seizure medication (ASM) therapy. At baseline, a minNPi ≤4.0 was found in 85.3% of patients with NCSE. After ASM therapy, 77.6% of the minNPi values were normalized in the treatment responder group among the NCSE-terminated patients (n=66). Moreover, the improvement in minNPi was significant according to the responsiveness of each ASM in responder groups [33]. Therefore, a quantitative pupillometer could be a useful non-invasive neuromonitoring tool for the diagnosis and treatment responsiveness of NCSE.
Other neurological diseases
Quantitative pupillometry could be a useful neurological monitoring tool for patients with cortical dysfunction for several medical reasons. In an observational study of liver transplantation (n=183), unconscious patients with grade 4 hepatic encephalopathy before liver transplantation had a prolonged latency phase and reduced constrictive ratio (the value of the resting diameter minus the maximum constriction diameter/resting diameter). In addition, the recovery of pupillometer parameters was slower in patients with grade 4 hepatic encephalopathy after liver transplantation, in which the recovery time of grade 4 hepatic encephalopathy was delayed to 36 hours. In contrast, other groups recovered within 24 hours of transplantation [34]. Moreover, lower %CH and CV in the early stages of intensive care unit (ICU) care were associated with the development of delirium irrespective of the baseline severity, analgesia, and sedation dose in sedated, mechanically ventilated ICU patients without brain injuries [35]. A prospective observational study based on ICU patients reported that a lower DV was independently related to unreactive EEG findings, indicating no EEG change in response to external stimuli in patients with brain injuries, such as stroke, traumatic brain injury, infection, and other medical diseases [41].
FACTORS AFFECTING THE PUPILLOMETER PARAMETERS IN THE NEUROLOGICAL INTENSIVE CARE UNITS
Several factors related to treatments and environments in the neuroICU are associated with pupillometer parameters. Many medications that affect the PLR are frequently used in neuroICUs. The most used medications are opioids, which usually induce miosis by activating parasympathetic activity. In healthy volunteers who received remifentanil, the drug reduced pupil diameters (5.6 mm to 2.5 mm) related to hypercarbia and hypoxia and decreased NPi values (4.6 to 4.3), but the values were within the normal range. Therefore, PLR examination during opioid administration may be useful for neurological assessment [42]. In the neuroICU, sedative drugs, such as benzodiazepine, propofol, and dexmedetomidine, are commonly used for treatment and sedation in neurocritically ill patients. In a prospective study of 15 male volunteers, pupillary function, including pupil diameter and light reflex function, was examined under darkness and at three luminance levels after oral administration of diazepam (10 mg), and pupil diameter and light reflex function did not change during monitoring [43]. Regarding the effect of propofol on PLR, a cross-sectional study of 19 volunteers treated with propofol reported that propofol sedation decreased pupil diameter and CV [44]. In patients with acute intracranial pathology, dexmedetomidine induced an increase in NPi (3.77 to 4.14) and smaller pupil diameters (3.41 to 3.13) despite the values being within the normal range [7]. PLR changes in the neuroICU could be affected by ambient light and circadian rhythms in the neuroICU. The circadian rhythm is changed by external stimuli such as abnormal lighting, nocturnal light exposure, noise, and altered feeding schedules. Therefore, pupil dynamics in the neuroICU may be altered by the circadian phases and environment of the neuroICU [45-47]. Additionally, age, iris color, and sex may affect PLR [48]. Although several factors, including medications and the environment, could affect PLR changes in the neuroICU, serial monitoring of PLR using a quantitative pupillometer is a helpful neuromonitoring method because the variability of PLR is within the normal range.
CONCLUSIONS
PLR has long been an important neurological examination for evaluating various neurological conditions. A quantitative pupillometer provides objective and reliable parameters for assessing changes in PLRs compared to manual pupil examinations. Quantitative pupillometry has many potential benefits in detecting intracranial pathology, treatment effects, and monitoring increased ICP in the neuroICU. Moreover, the parameters of the quantitative pupillometer have prognostic value when assessing outcomes after CA. Although PLR is affected by several factors, such as medications and environments, quantitative pupillometry could be an important clinical monitoring tool to evaluate changes in neurological conditions for determining treatment effects and outcomes in the neuroICU.
Notes
Ethics statement
Not applicable.
Conflict of interest
Tae Jung Kim is an editorial board member of the journal, but she was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Acknowledgments
This study was funded by Seoul National University Hospital (No. 0320210350).