In-hospital mortality of atrial fibrillation-associated acute ischemic stroke in the intensive care unit
Article information
Abstract
Background
Although atrial fibrillation (AF)-associated acute ischemic stroke (AIS) is on the rise, is devastating, and life-threatening, there is limited data on the clinical course and in-hospital mortality of patients treated in the intensive care unit (ICU). This study aimed to describe the clinical course and factors associated with in-hospital mortality in AF-associated AIS patients admitted to the ICU.
Methods
This study was a retrospective analysis of a prospective nationwide multicenter cohort including non-valvular AF-AIS patients receiving ICU care admitted to 14 stroke centers in South Korea from 2017 to 2020. In-hospital outcomes, including in-hospital mortality and neurological deterioration (ND) have been described.
Results
Amongst 2,487 AF-associated AIS patients, 259 (10.4%) were treated in the ICU. In-hospital mortality and ND occurred in 8.5% and 17.0% of the patients, respectively. Higher rates of initial National Institute for Health Stroke Scale scores, symptomatic steno-occlusive lesions, and CHA2DS2-VASc (Congestive Heart Failure, Hypertension, Age ≥75 [Doubled], Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack [Doubled], Vascular Disease, Age 65–74, Female) scores were found in those with in-hospital mortality. The CHA2DS2-VASc score after admission increased the risk of in-hospital mortality (odds ratio [OR], 1.48; 95% confidence interval [CI], 1.00–2.18) were associated with in-hospital mortality. Antithrombotic use within 48 hours was related to decreased in-hospital mortality (OR, 0.26; 95% CI, 0.10–0.67).
Conclusion
ICU care in AF-associated AIS is common, and the establishment of optimal treatment strategies in the ICU may be needed.
INTRODUCTION
Atrial fibrillation (AF) is a major risk factor for ischemic stroke, contributing to an incremental risk of more than five times [1]. Furthermore, AF was associated with more severe symptoms and a greater than 30-day mortality risk among acute ischemic stroke (AIS) patients as per the Framingham sub-study [2]. As AF prevalence increases with age from 0.1% in those aged <55 years to 9.0% in those aged 80 years or older [3], the number of AF-related embolic events is estimated to triple by 2050 with an increasing average life span [4]. Therefore, discussions on treatment strategies for this devastating, life-threatening, and increasing AF-associated AIS is essential to improve patient care [5].
Proper management in the intensive care unit (ICU) is known to improve outcomes in neurological diseases [6]. For AIS, ICU care is focused on post-reperfusion management, cerebral edema/increased intracranial pressure (IICP) treatment, determination of surgical options, prevention of stroke progression and recurrence, and airway/respiratory support [7,8]. If AF-AIS patients have greater infarct size, infarct growth, and hemorrhagic transformation rates [9], dedicated ICU care for the indicated AF-AIS patients is essential, and the role of ICU care should especially be highlighted in them. However, data regarding AF-AIS patients treated in the ICU are scarce.
Understanding individual profiles and clinical courses may be required to establish optimal treatment strategies to enhance outcomes in AF-AIS patients in the ICU. In this study, we aimed to describe baseline characteristics and stroke information in AF-associated AIS patients treated in the ICU, compared to those who did not; further, the clinical parameters associated with in-hospital mortality using clinical data from a prospective nationwide multicenter AF cohort study were investigated.
METHODS
Study subjects
Among AIS patients admitted to 14 stroke centers in Korea, the East Asian Ischemic Stroke Patients with Atrial Fibrillation Study (EAST-AF) Part II was used to provide risk stratification tools for assessing the risk of stroke recurrence by collecting clinical and neuroimaging characteristics potentially associated with clinical outcomes. The EAST-AF Part II prospectively enrolled patients with nonvalvular AF. These patients included those with priorly known AF and AF diagnosed after stroke upon routine electrocardiography, automatic electrocardiography monitoring or 24-hour Holter monitoring during their hospital stay. Clinical information and outcome data were derived from the Clinical Research Collaboration for Stroke in Korea (CRCS-K) registry [10].
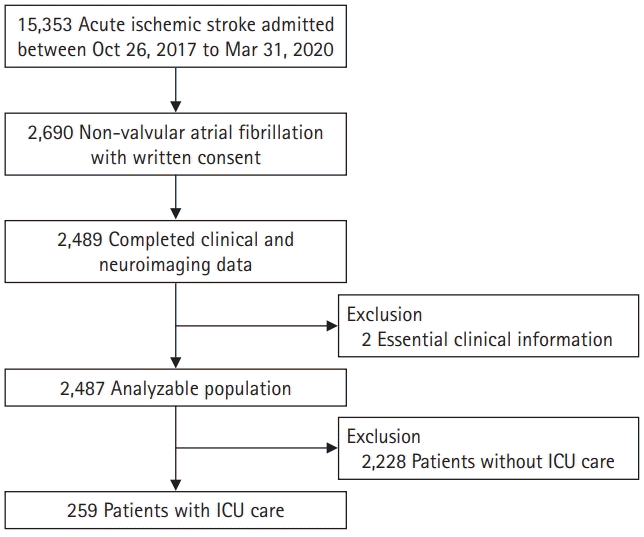
A total of 15,353 patients admitted to the EAST-AF-Part II participating centers between October 26, 2017, and March 31, 2020, were screened. Amongst 2,690 non-valvular AF patients who provided informed consent, we included 2,489 patients who completed clinical and neuroimaging data from the prospective registry in this study (Fig. 1). After excluding two patients with essential clinical information, 2,487 patients were included in the analysis. In total, 259 ICU patients were enrolled in the current study. ICU admission was determined by neurological (malignant middle cerebral artery infarction, stroke causing decreased consciousness, in need of treatment for increased intracranial cerebral pressure or monitoring, etc.), cardiopulmonary (cardiac arrest, heart failure, pneumonia, pulmonary embolism, acute respiratory distress syndrome requiring intubation and ventilator support), and other clinical conditions. Physicians determined the need for ICU care [6,11].
Data collection and outcome assessment
Clinical data were obtained from the CRCS-K database, including records of intensive care during hospital stay. Information on sex, age, vascular risk factors including hypertension, diabetes, dyslipidemia, smoking status, history of stroke and coronary heart disease, and heart failure was further collected. Data on prior antithrombotic and premorbid functional statuses were also collected. Stroke information such as systolic and diastolic blood pressure, initial glucose level, initial National Institute for Health Stroke Scale (NIHSS) score, symptomatic steno-occlusive lesion (>50% stenosis or occlusion) [12], emergent revascularization therapy (intravenous thrombolysis and endovascular treatment [EVT]), and acute antithrombotic treatment including antiplatelet and anticoagulant therapy within 48 hours of admission were evaluated. Door-to-needle time was defined in 89 patients who underwent intravenous thrombolysis and an onset-to-arrival of <24 hours. Door-to-puncture time was defined in 99 patients who underwent EVT and an onset-to-arrival of <24 hours, and onset-to-reperfusion time was defined in 79 patients who underwent EVT, ≥2a thrombolysis in cerebral infarction, and an onset-to-arrival of <24 hours.
The primary outcome was in-hospital mortality rate. Among the three discharge states, in-hospital mortality, transfer to other departments, and discharge, the occurrence of in-hospital mortality was analyzed. In-hospital mortality included patients with hopeless discharge. The occurrence of neurological deterioration (ND) was also assessed. ND was defined as any new neurological symptoms or signs worsening among patients with a total NIHSS score ≥2 or an increase in the NIHSS subscore of ≥1 for consciousness or motor function level, occurring during the hospital stay within 3 weeks of onset [13]. Stroke recurrence, stroke progression, symptomatic hemorrhagic transformation, and other causes including myocardial infarction, pulmonary embolism, deep vein thrombosis, or unknown etiologies constituted ND. Stroke recurrence was determined as having new discrete lesions on brain imaging. Stroke progression was defined as neurologic deterioration lasting more than 24 hours due to progressive ischemia, swelling, or perilesional edema of the infarcted area, distinguished from stroke recurrence with brain imaging, which is caused by new discrete lesions [14]. Amongst stroke progression, brain swelling/IICP was defined as ND caused by swelling of infarcted tissue or perilesional edema that was confirmed by a physician with brain imaging. Symptomatic hemorrhagic transformation was diagnosed as per European Cooperative Acute Stroke Study (ECASS) criteria, defined as any hemorrhage site found on brain imaging that caused a decrease in the NIHSS score of ≥4 [15,16]. The modified Rankin scale scores at discharge and number of admission days were also obtained. The change in NIHSS score from the initial NIHSS score to the discharge NIHSS score was calculated.
Statistical analysis
Baseline characteristics and stroke information of patients in the ICU were described as mean±standard deviation or median (interquartile range [IQR]), as appropriate. The discharge status and proportion of ND were described. Patients who received ICU care were further distinguished based on the occurrence of in-hospital mortality. We compared the baseline characteristics and stroke information between patients with and without in-hospital mortality. Clinical parameters that differed between the two groups were determined using the chi-square test or Fisher’s exact test for categorical variables and Student t-test or Mann Whitney U-test for continuous variables, as appropriate. Among variables of P<0.05 in difference between the two groups, the relationship between in-hospital mortality and clinical parameters in difference was analyzed by binary logistic regression model. We established multivariable models as follows: (1) unadjusted model and (2) adjusted model with the initial NIHSS score. With substantial validated predictability, the initial NIHSS score was chosen as a variable for adjustment [17]. Due to the limited number of outcomes, the variables were adjusted with the initial NIHSS score. If the CHA2DS2-VASc (Congestive Heart Failure, Hypertension, Age ≥75 [Doubled], Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack [Doubled], Vascular Disease, Age 65–74, Female) score was related to the outcome, variables that were components of the CHA2DS2-VASc score were not included in the multivariable model, considering potential multicollinearity. Multicollinearity between the initial NIHSS score and the variables was evaluated using the variance inflation factor, and no significant relationship was found. Odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated. Data were analyzed using R 4.1.3, and a P-value of <0.05 was considered statistically significant.
RESULTS
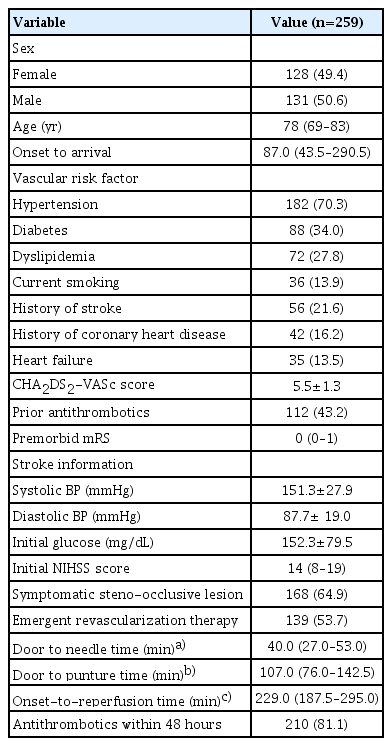
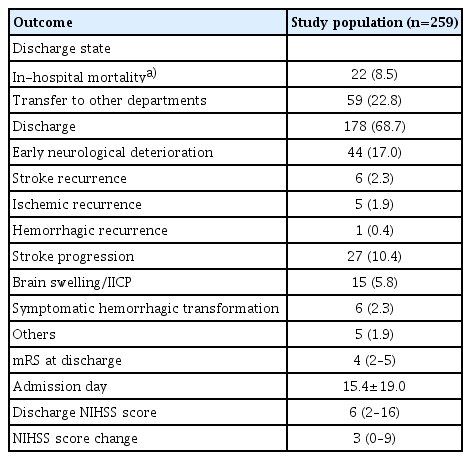
Demographics and stroke information were described for patients receiving ICU care (Table 1). The median age was 78 (IQR, 69–83) and the median CHA2DS2-VASc score was 5.5±1.3. Antithrombotics prior to the index stroke were used in 43.2% of patients. The median NIHSS score was 14 (IQR, 8–19) and 53.7% of the patients underwent emergent revascularization therapy. Among the AF-associated AIS patients treated in the ICU, in-hospital mortality occurred in 22 patients (8.5%) (Table 2). Two-thirds of the patients were discharged. ND occurred in 17.0% of patients. Stroke progression occurred in 10.4% of the patients and was the most frequent subtype of ND, which included 5.8% of the patients whose ND was caused by brain swelling or increased intracranial cerebral pressure. Symptomatic hemorrhagic transformation was observed in 2.3% of patients. After admission, the median decrease in the NIHSS score was 3.
Baseline characteristics of atrial fibrillation-associated acute ischemic stroke patients with intensive care management
In-hospital outcomes of atrial fibrillation-associated acute ischemic stroke patients treated in the intensive care unit
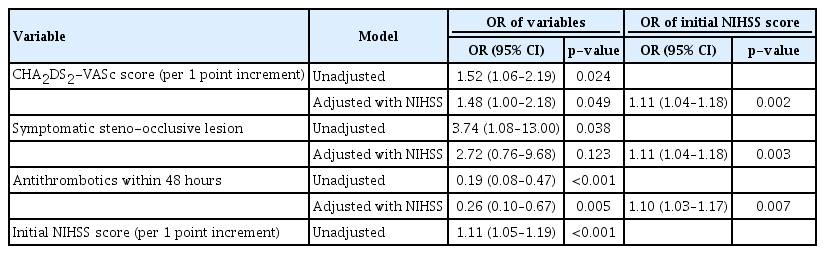
Comparing patients without in-hospital mortality among AF-associated stroke in the ICU, patients with in-hospital mortality were older, had higher CHA2DS2-VASc score, initial NIHSS score, proportion of symptomatic steno-occlusive lesions, and lower acute antithrombotic treatment within 48 hours (Table 3). In the logistic regression model, the initial NIHSS score increased the odds of in-hospital mortality in the unadjusted model (OR, 1.11; 95% CI, 1.05–1.19) (Table 4). When adjusted for the initial NIHSS score, CHA2DS2-VASc score (OR, 1.48; 95% CI, 1.00–2.18]) increased the risk of in-hospital mortality, while symptomatic steno-occlusive disease (OR, 2.72; 95% CI, 0.76–9.68) did not. Antithrombotic use 48 hours after admission was associated with a low mortality risk (OR, 0.26; 95% CI, 0.10–0.67).
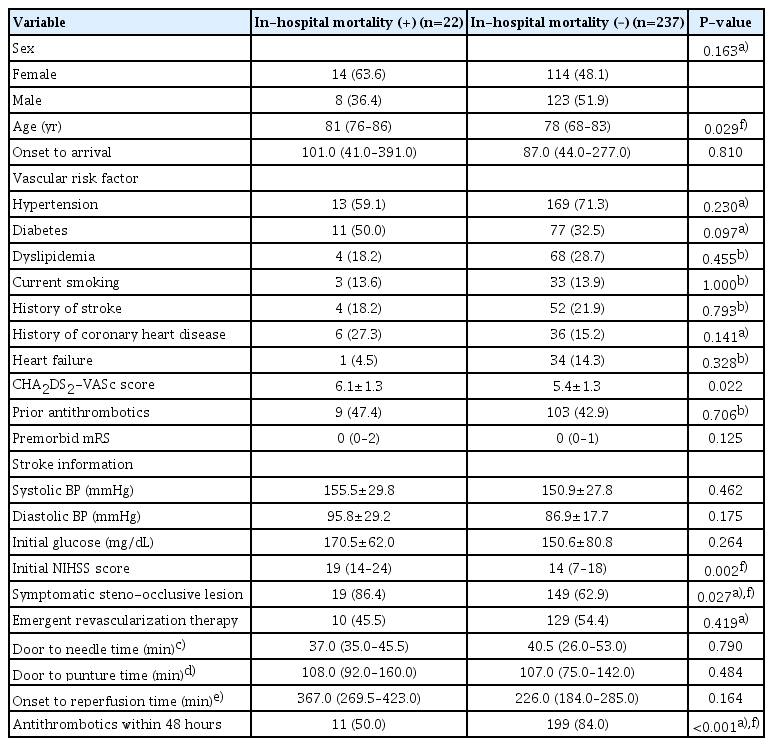
Comparison of baseline characteristics and outcomes in atrial fibrillation-associated acute ischemic stroke patients treated in the intensive care unit according to in-hospital mortality
DISCUSSION
In this retrospective analysis of a multicenter prospective cohort of AF-associated AIS patients, approximately one-tenth of the patients were managed in the ICU. The CHA2DS2-VASc score was associated with increased in-hospital mortality, whereas antithrombotic treatment within 48 h was related to low in-hospital mortality. ND and stroke progression, including brain swelling, were frequently observed in these patients. A decrease in the NIHSS score from admission to discharge was observed.
Several studies on AIS patients receiving ICU care have reported variable hospital mortality and functional outcomes [11,18]. Compared with previous literatures (25%–40%), the in-hospital mortality rate was quite low (8.5%). This finding might be attributable to the variable indications of ICU admission according to hospital policy or physicians’ decisions. An observational study of neurological and neurosurgical ICU in Korea reported similar in-hospital mortality (7.3% for ICU and 4.7% for neurosurgical ICU), comparable to the current study [6]. This study also suggests the potential benefit of ICU care with the improvement of NIHSS score of 3 points at discharge, and the rates of patients with an indication of neurological treatment. As neurological aspects of ICU care in AIS patients concentrate on post-reperfusion therapy and ND [7,8,19,20], this study reported that emergent revascularization therapy reported in 53.7% of ICU-treated patients could be a potential target population for the neurological management in the ICU.
Several clinical parameters have been associated with in-hospital mortality in patients with ICU-treated AF-associated AIS. The CHA2DS2-VASc score is a well-established risk stratification tool for stroke and thromboembolism in AF [21]. CHA2DS2-VASc score has been reported as an independent value to predict long-term mortality in AF patients [22]. The current study suggests that the CHA2DS2-VASc score could be utilized for predicting the in-hospital mortality of ICU-treated AF-associated AIS patients, and a meticulous inspection of adverse events in patients with high scores might be needed to enhance in-hospital outcomes. Symptomatic steno-occlusive lesions showed a tendency to increase mortality risk without statistical significance in the current study. In the previous literature, symptomatic steno-occlusive lesions were associated with unfavorable functional outcomes in AIS patients [22]. In the era of EVT for AIS patients [23], treatment strategies for the failed recanalization cases might be developed in neurocritical care, which constituted 86% of the expired patients in the current study. Furthermore, although EVT was performed in the indicated patients, a treatment plan for obtained recanalization with a large infarct core is also needed, as AF is one of the risk factor for futile recanalization following EVT [24].
The lower risk of mortality in patients receiving acute antithrombotic treatment might be attributable to the preventive effect as well as selection bias. Acute antithrombotic therapy has been proven to reduce the risk of stroke recurrence and has been applied to AIS patients in current practice [25,26]. However, a discrepancy in stroke severity (median initial NIHSS score of 19 vs. 14 in patients who died and survived, respectively) infers a difference in neurologic and medical conditions between the two groups. Therefore, a cautious interpretation is needed for acute antithrombotic treatment in patients with ICU-treated AF-associated AIS. In the acute phase of AF-associated ischemic stroke, physicians often face a great dilemma in initiating anticoagulation and optimal timing of commencement due to the risk of hemorrhagic transformation [27]. As a larger infarction is a strong predictor of hemorrhagic transformation in AF-associated stroke [28], guidelines on starting anticoagulation therapy recognize a distinction in their recommendations according to stroke severity [29,30]. Ongoing trials on anticoagulation timing are expected to provide high-level of evidence with safety and efficacy profiles [31,32]. A thoughtful risk-benefit balance in initiating anticoagulation therapy based on individual clinical situations is required for severe AF-associated AIS patients.
This study has several strengths. To our knowledge, this is the largest prospective AF cohort study in Asia, consisting of 14 nationwide stroke centers. This cohort represents the current clinical status and real-life practice of AF stroke management in Korea. As we collected data from a nationwide multicenter prospective cohort, we also attempted to reduce bias in the enrollment of participants. This cohort had a high outcome capture rate (3-month capture rate, 99%), on which this study could provide relatively accurate outcome information.
However, this study has several limitations. First, as decisions of ICU admission vary according to the centers’ policy in the indication, medical resources, and physicians’ opinions, the variable effect of the center or physician might be present. Detailed information on the indication for ICU admission and the time from onset to ICU admission were not available. Second, some patients with irreversible neurological damage with very severe stroke or underlying incurable progressive diseases, including malignancy, could have rejected ICU care, but resulted in in-hospital mortality and might not have been included in this study. Third, specific echocardiographic findings, such as left atrium diameter or cardiac markers, including brain natriuretic peptide or cardiac enzymes, were not included in the analysis. Further studies involving specific cardiac markers are warranted in the future.
In conclusion, ICU care is common in patients with AF-associated ischemic stroke. Initial stroke severity and CHA2DS2-VASc score increased the risk of in-hospital mortality whereas antithrombotic treatment was associated with decreased risk. To improve patient outcomes in AF-associated AIS, establishing optimal treatment strategies with upcoming high-level evidence may be required.
Notes
Ethics statement
The study was reviewed and approved by the Institutional Review Boards of the participating centers (No. B-1705/396-306). Written informed consent was obtained from all patients.
Conflict of interest
No potential conflict of interest relevant to this article.
Author contributions
Conceptualization: BKK. Data curation: DYK, BKK. Formal analysis: DYK, BKK. Investigation: HGJ, CYP, JYK, BJK. Methodology: HGJ, CYP, JYK, BJK. Project administration: DYK, HJB, BKK. Resources: all authors. Software: all authors. Supervision: JK, JYK, MKH, HJB, BKK. Validation: JK. HGJ, CYP, JYK, BJK, MKH, HJB, BKK. Visualization: DYK, JK, CYP, HJB, BKK. Writing–original draft: DYK, CYP, HJB, BKK. Writing–review & editing: JK, CYP, JYK, BKK.
Additional contributions
We would like to express our gratitude to the institutions that provided us with the opportunity to analyze the East Asian Ischemic Stroke Patients with Atrial Fibrillation Study (EAST-AF) and Clinical Research Collaboration for Stroke in Korea (CRCS-K) databases for this study.
The CRCS-K investigators
Jun Yup Kim, MD; Jihoon Kang, MD, PhD; Beom Joon Kim, MD, PhD; Moon-Ku Han, MD, PhD; Hee-Joon Bae, MD, PhD (Seoul National University Bundang Hospital); Kang-Ho Choi, MD; Joon-Tae Kim, MD, PhD; Man-Seok Park, MD, PhD; Ki-Hyun Cho, MD, PhD (Chonnam National University Hospital); Kyu Sun Yum, MD; Dong Ick Shin, MD, PhD (Chungbuk National University Hospital); Dae-Hyun Kim, MD, PhD; Jae-Kwan Cha, MD, PhD (Dong-A University Hospital); Dong-Seok Gwak, MD; Wi-Sun Ryu, MD, PhD; Dong-Eog Kim, MD, PhD (Dongguk University Ilsan Hospital); Jong-Moo Park, MD, PhD (Uijeongbu Eulji Hospital); Yong Soo Kim, MD; Kyusik Kang, MD, PhD (Eulji General Hospital); Jae Guk Kim, MD; Soo Joo Lee, MD, PhD (Eulji University Hospital); Minwoo Lee, MD; Mi-Sun Oh, MD, PhD; Kyung-Ho Yu, MD, PhD; Byung-Chul Lee, MD, PhD (Hallym University Sacred Heart Hospital); Hong-Kyun Park, MD; Yong-Jin Cho, MD, PhD; Keun-Sik Hong, MD, PhD (Inje University Ilsan Paik Hospital); Chul-Hoo Kang, MD; Joong-Goo Kim, MD; Jay Chol Choi, MD, PhD (Jeju National University Hospital); Jang Seong Hwa, MD; Hyungjong Park, MD; Jeong-Ho Hong, MD, PhD; Sung-Il Sohn, MD, PhD (Keimyung University Dongsan Medical Center); Tai Hwan Park, MD, PhD; Sang-Soon Park, MD (Seoul Medical Center); Wook-Joo Kim, MD; Jee-Hyun Kwon, MD, PhD (Ulsan University Hospital); Kyung Bok Lee, MD, PhD (Soonchunhyang University Hospital); Kwon Doo Hyuk, MD; Jun Lee, MD, PhD (Yeungnam University Medical Center); Keon-Joo Lee, MD (Korea University Guro Hospital); Sang-Hwa Kee, MD, PhD; Chulho Kim, MD, PhD (Hallym University Chuncheon Sacred Heart Hospital); Hae-Bong Jeong, MD; Kwang Yeol Park, MD, PhD (Chung-Ang University Hospital); Ji Sung Lee, PhD (Asan Medical Center), Juneyoung Lee, PhD (Korea University)