INTRODUCTION
Takotsubo syndrome is characterized by transient and reversible left ventricular (LV) dysfunction and is typically triggered by emotional or physical stress [1]. Myasthenic crisis can also be associated with emotional or physical stress [2]. A small number of cases have been reported describing a combination of Takotsubo syndrome and myasthenic crisis. We present the case of a woman with myasthenia gravis who developed Takotsubo syndrome and myasthenic crisis following radiocontrast media-induced anaphylaxis.
CASE REPORT
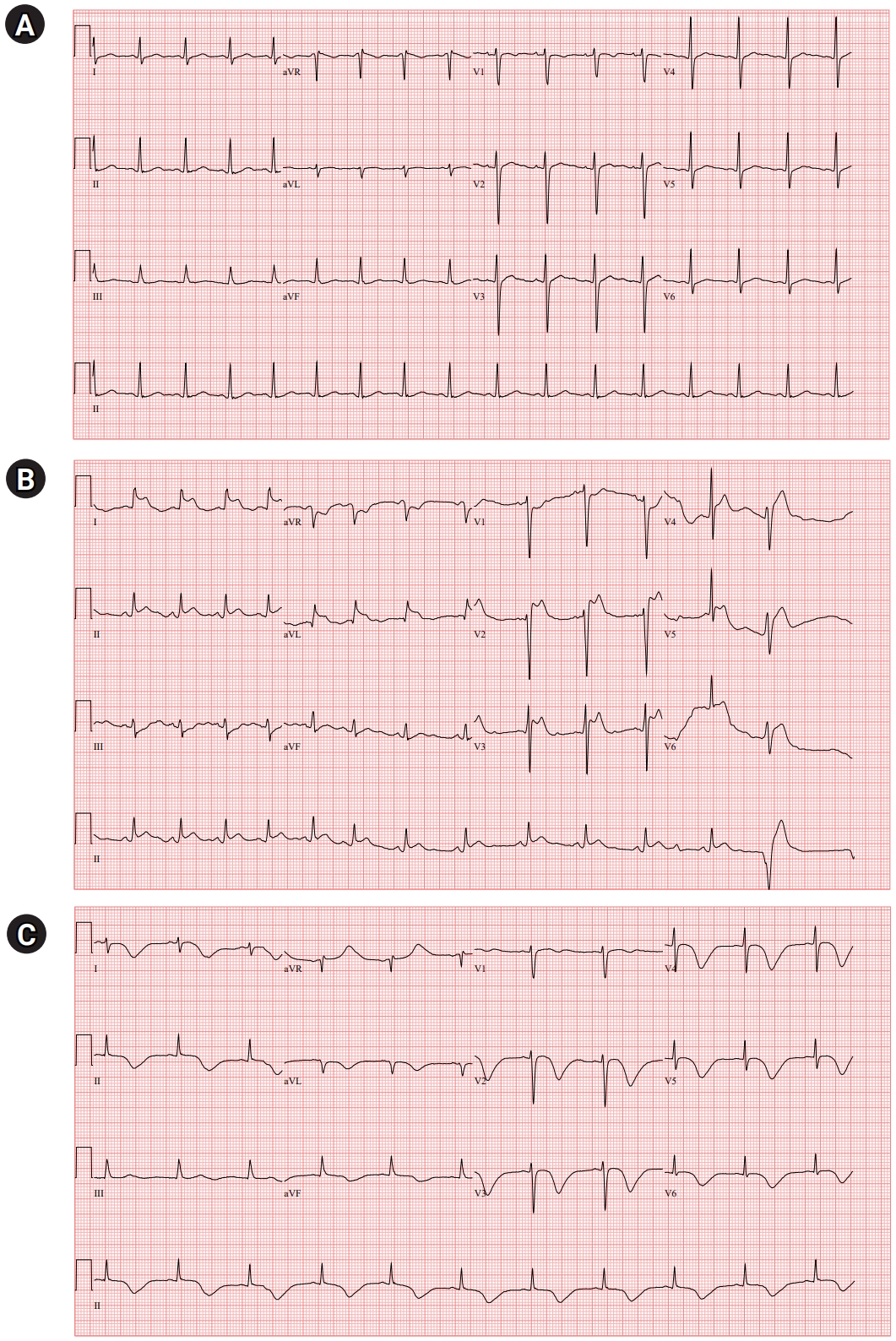
A 39-year-old woman presented with diplopia and ptosis that worsened in the evening. She had dysarthria, dysphagia, mastication difficulty, limb and neck weakness, and mild dyspnea that had progressed for 3 weeks. She had no history of major illness and was not taking any medications. She had no allergies to any medicines, food, or environmental allergens. Neurological examination revealed bilateral ptosis with fatigability and bilateral medial gaze limitation. The muscle powers of the limbs were Medical Research Council (MRC) grade 4/5. The strengths of neck flexion and extension were MRC grade 4/5 and MRC grade 3/5, respectively. The sensory function was normal, deep tendon reflex was normoactive, and no pathological reflexes were observed. On admission, her vital signs and routine blood investigations were normal. A 12-lead electrocardiogram (ECG) revealed normal findings with normal sinus rhythm (Fig. 1A).
After a computed tomography (CT) scan of the chest, she complained of chest tightness while being transferred to a wheelchair and suddenly lost consciousness. Her consciousness was stupor, and her oxygen saturation decreased to 80%. After manual resuscitation with a bag valve mask, she could obey simple commands but again lost consciousness. An arterial blood gas showed respiratory acidosis with hypercapnia (PH, 7.139; PCO2, 77.5 mmHg; PO2, 74.2 mmHg; and HCO3, 26.3 mmol/L). Epinephrine 0.3 mg was injected intramuscularly, and intubation was performed. ECG showed ST elevation in lead I, II, aVL, and V2ŌĆō5 (Fig. 1B); however, it was incompatible with the coronary artery territory. Creatine kinases-myocardial band (CK-MB) was 1.8 ng/mL (normal <5.0 ng/mL) and troponin I was 0.008 ng/mL (normal <0.047 ng/mL). She was mechanically ventilated and transferred to the intensive care unit. Sedatives with midazolam and remifentanil and inotropic with norepinephrine were infused. Intravenous heparin and dual antiplatelet therapy were initiated.
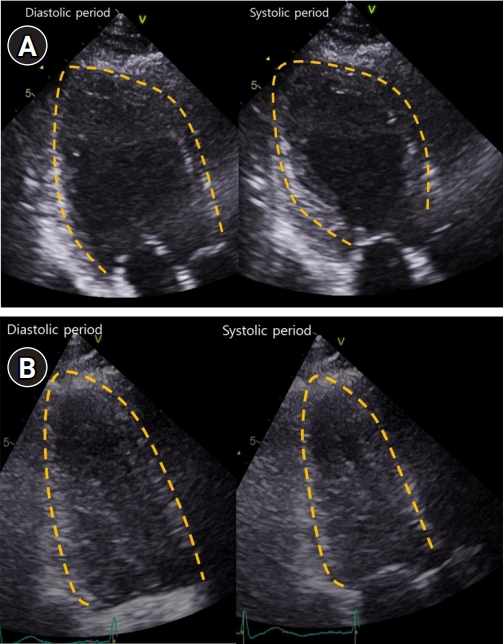
On the next day, transthoracic echocardiography (TTE) demonstrated severe global hypokinesia of the LV base, akinesia of the mid-LV to the apex without thinning, and reduced LV systolic function with LV ejection fraction of 38% (Fig. 2A). Serial ECG revealed dynamic changes with marked T-wave inversion and prolonged corrected QT interval (QTc) interval, peaking at 612 ms (Fig. 1C). CK-MB and troponin I levels were elevated, peaking at 10.9 ng/mL and 4.65 ng/mL, respectively. She did not undergo cardiac catheterization because she experienced anaphylaxis after exposure to radiocontrast media.
A diagnosis of myasthenia gravis was made based on clinical signs, positive ice pack test, elevated anti-acetylcholine receptor antibody titer (17.80 nmol/L) and significant decrement on repetitive nerve stimulation test with low frequency rates (41.5% in orbicularis oculi, 71.3% in nasalis, 27.2% in abductor digiti minimi, 50.9% in flexor carpi ulnaris, and 73.8% in trapezius). Chest CT showed about 3.3-cm sized lobulated mass at the anterior mediastinum.
Extubation failed, and her weakness worsened. The myasthenic crisis was diagnosed as superimposed on Takotsubo syndrome. High-dose intravenous methylprednisolone pulse therapy was started on day 3 of hospitalization, and prednisolone (1 mg/kg/day) was administered after methylprednisolone pulse therapy. Her neurological condition gradually improved; she was weaned off the ventilator and extubated on day 15 of hospitalization. Repeat TTE 6 and 13 days after the initial TTE demonstrated an improved LV ejection fraction of 45% and 55%, respectively (Fig. 2B). The patient was discharged on day 32 of hospitalization.
DISCUSSION
Takotsubo syndrome, also known as Takotsubo cardiomyopathy, stress-induced cardiomyopathy, broken heart syndrome, or apical ballooning syndrome, is characterized by transient and reversible LV dysfunction. Takotsubo syndrome is typically triggered by emotional or physical stress, although approximately one-third of patients present with no identifiable stressful triggers. Takotsubo syndrome is similar to acute coronary syndrome in terms of symptoms at presentation, ECG abnormalities, elevated cardiac biomarkers, and comparable in-hospital mortality [1]. The most common symptoms of Takotsubo syndrome are chest pain, dyspnea, or syncope. ECG abnormalities in Takotsubo syndrome include ST-segment elevation, ST-segment depression, symmetric T-wave inversion, and QTc prolongation. Cardiac troponin T and CK-MB levels are elevated in most patients with Takotsubo syndrome, but the biomarkers rise only slightly compared to the extensive wall motion abnormalities in the early stages. LV regional wall motion abnormalities extend beyond the distribution of a single epicardial coronary artery, and no significant obstructive coronary artery disease that can explain the extent of LV dysfunction has been found. Although echocardiography is the first-line imaging tool for patients with suspected Takotsubo syndrome, coronary angiography plays a crucial role in ruling out alternative diagnoses, and ventriculography may show a variety of LV regional wall motion abnormalities [1,3]. Takotsubo syndrome is thought to have a benign course, but recent studies have shown that major short-term complications, such as cardiogenic shock and mortality, are comparable to those of acute coronary syndrome. LV dysfunction in Takotsubo syndrome usually resolves spontaneously within days or weeks [3]. This patient did not undergo cardiac catheterization, but was diagnosed with Takotsubo syndrome due to typical disease features. ECG showed ST elevations that were not compatible with coronary artery territory, echocardiography showed LV regional wall motion abnormalities that extended beyond the distribution of a single epicardial coronary artery and the appearance of apical ballooning, and CK-MB and troponin T were only slightly elevated compared to extensive wall motion abnormalities. Most importantly, LV dysfunction resolved spontaneously within 2 weeks.
In anaphylaxis, the heart, and especially the coronary arteries, are the primary targets of inflammatory mediators [4]. Cases of Takotsubo syndrome triggered by anaphylaxis have been reported [5,6]. Elevated catecholamines and excessive activation of cardiac catecholamine receptors in the left ventricle play a major role in the pathophysiology of Takotsubo syndrome. In anaphylaxis, catecholamines are released by the renin-angiotensin-aldosterone system and histamine, and catecholamines administered as treatment for anaphylaxis would also increase plasma catecholamine levels [7]. In this patient, Takotsubo syndrome was likely triggered by the critical physiological state from the anaphylaxis and subsequent administration of exogenous catecholamine (0.3 mg intravenous epinephrine).
According to the World Allergy Organization, the diagnostic criteria for anaphylaxis are (1) typical skin symptoms and significant symptoms from at least one other organ system, or (2) exposure to a known or probable allergen for that patient, with respiratory and/or cardiovascular compromise. The most frequent anaphylaxis elicitors are food, insect venom, and drugs. Other elicitor groups include natural rubber latex, seminal fluid, radiocontrast media, and medical dyes [8]. The diagnosis of anaphylaxis in this patient was made by chest tightness, dyspnea, and hypoxemia that occurred suddenly after exposure to radiocontrast media within minutes. Myasthenic crisis is a complication of myasthenia gravis, characterized by worsening muscle weakness and respiratory failure. Patients with myasthenic crises typically experience increased generalized weakness as a warning. Since this patient showed respiratory compromise that occurred suddenly after a CT scan, it appears that she had respiratory failure due to anaphylaxis, not a myasthenic crisis.
Myasthenic crisis is often triggered by stressors, such as infection, surgery, pregnancy, childbirth, or various medications. Other antecedent factors include exposure to temperature extremes, pain, sleep deprivation, and physical or emotional stress [2]. The next day after anaphylaxis, extubation failed and her weakness worsened. Myasthenic crisis was thought to be superimposed on Takotsubo syndrome. A small number of cases have been reported describing a combination of these two diseases, all of which are Takotsubo syndrome associated with myasthenic crisis [9,10]. Takotsubo syndrome following myasthenic crisis had a 15-fold higher prevalence, nearly two-fold higher all-cause mortality, and higher resource utilization than Takotsubo syndrome caused by other etiologies [10]. According to the international consensus guidelines for the management of myasthenia gravis, older reports of iodinated radiocontrast agents document increased myasthenia gravis weakness, but modern radiocontrast agents appear to be safer. It is recommended to use them cautiously and observe worsening [11]. The use of modern, low-osmolality agents has been reported with conflicting results with respect to worsening myasthenic symptoms [12,13]. The triggers of myasthenic crisis in these patients could be complex with the physical stress of anaphylaxis and Takotsubo syndrome, emotional stress from a serious physical condition, and radiocontrast agents.
Kounis syndrome, also known as allergic angina, allergic myocardial infarction, or coronary hypersensitivity disorder, is defined as the concurrence of acute coronary syndromes with conditions associated with mast cell and platelet activation. Cardiac symptoms and signs, ECG, and laboratory findings were similar in Takotsubo syndrome and Kounis syndrome. Complete resolution of LV dysfunction within a few weeks is also characteristic of both syndromes [14]. Indeed, both syndromes can be induced by anaphylaxis. Kounis syndrome can be induced by an allergy, hypersensitivity, or anaphylaxis, and cases of Takotsubo syndrome triggered by anaphylaxis have been reported [5,6]. In addition, it is noteworthy that Takotsubo syndrome can coexist with or follow Kounis syndrome [15]. Differential diagnosis of Takotsubo syndrome or Kounis syndrome in this patient can be challenging, and the possibility that Takotsubo syndrome develops after Kounis syndrome due to anaphylaxis cannot be excluded. We report this case as Takotsubo syndrome after anaphylaxis because it is a widely recognized and well-established disease, but Kounis syndrome has been established by a limited research group and only a small number of cases have been reported.
In summary, we report a case of myasthenia gravis that developed Takotsubo syndrome and myasthenic crisis following radiocontrast media-induced anaphylaxis. Takotsubo syndrome can occur in patients with neurological disorders; therefore, neurologists need to know about this disorder. The combination of Takotsubo syndrome and myasthenic crisis is rare, but may be associated with a poor prognosis. It is unclear whether the worsening of myasthenic symptoms is related to the use of radiocontrast agents, but caution is recommended for use in patients with myasthenia gravis.