INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect any organ in the body. Neuropsychiatric involvement in SLE is common, and its estimated prevalence ranges from 37% to 95% [1]. The diagnosis of neuropsychiatric SLE (NPSLE) was proposed by American College of Rheumatology in 1999 for 19 categories of NPSLE that included central nervous system manifestations, such as aseptic meningitis, cerebrovascular disease, demyelinating disease, headache, movement disorder, myelopathy, seizure disorder, cognitive dysfunction, mood disorder, and psychosis, as well as peripheral nervous system manifestations, such as acute inflammatory demyelinating polyradiculoneuropathy, autonomic disorder, mononeuritis, myasthenia gravis, cranial neuropathy, plexopathy, and polyneuropathy [2]. More recently, neuropsychiatric syndromes such as cerebral venous thrombosis, posterior reversible encephalopathy syndrome (PRES), optic neuritis, progressive multifocal leukoencephalopathy, and idiopathic intracranial hypertension have been added to the classification of NPSLE [3,4]. As stated above, the clinical manifestations of NPSLE are diverse and heterogeneous, and NPSLE remains a diagnostic and therapeutic challenge [3]. Furthermore, the occurrence of similar phenomena because of steroid treatment or concomitant diseases such as hypertension and hyperlipidemia makes the diagnosis of NPSLE more difficult [4].
Lupus cerebritis is a poorly defined disease entity, described by some researchers as a former term for NPSLE [3]. However, others have referred the evidence of cerebral parenchymal damage with cerebrospinal fluid (CSF) inflammation in the absence of stroke and vasculitis as lupus cerebritis [5]. Herein, we report a severe case of lupus cerebritis that was treated successfully with rituximab.
CASE REPORT
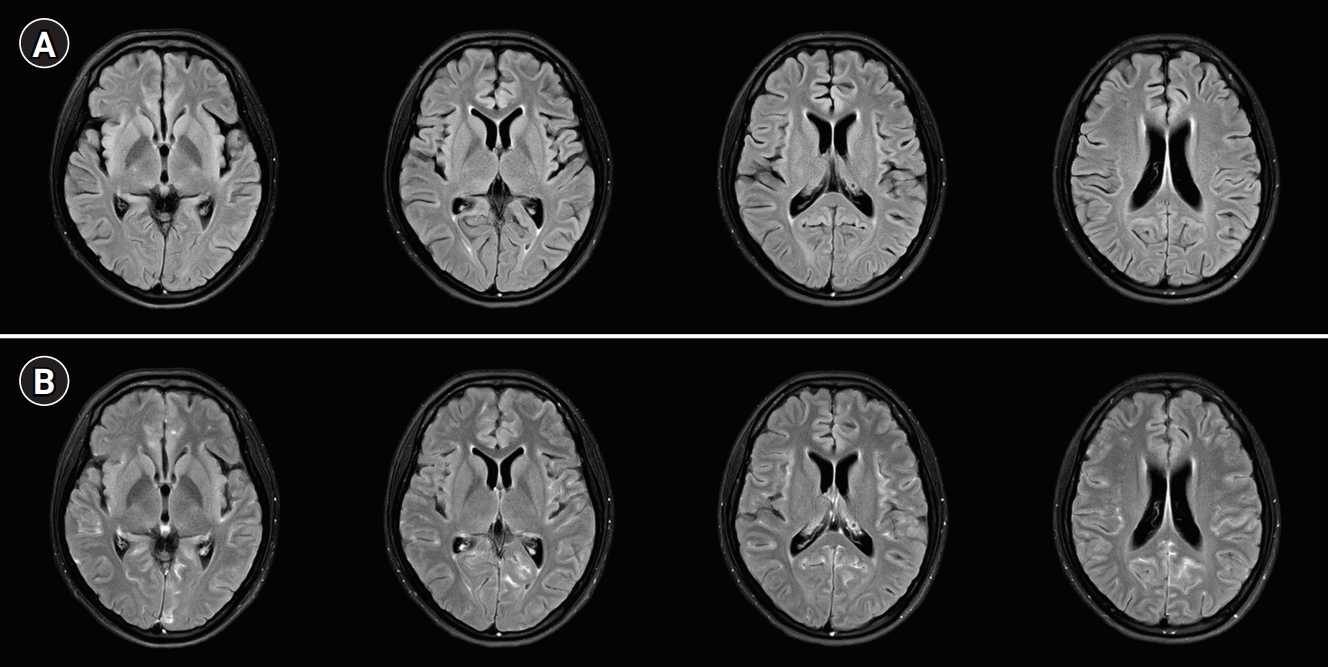
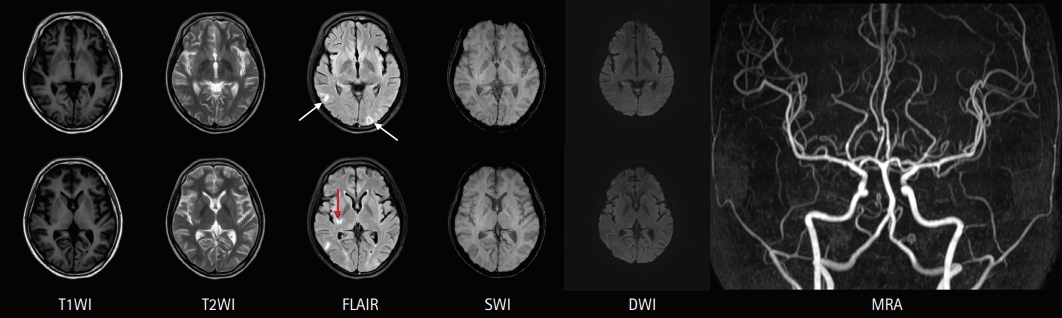
A 19-year-old woman presented to the emergency department with fever, headache, nausea, quadriparesis, and tremor. She had been diagnosed with SLE 19 months previously with the findings of discoid rash, non-erosive arthritis, leukopenia, low complement level, positive anti-dsDNA, positive anti-Smith antibody, and negative antiphospholipid antibodies, and she was treated with oral prednisolone at a dose of 15 mg/day and hydroxychloroquine at a dose of 200 mg/day. She had been experiencing inattention, anxiety, insomnia, compulsive behavior (frequent hand washing), tremor, and apraxia for 10 days before admission. Neurologic examination revealed decreased muscle strength in all extremities (Medical Research Council [MRC] grade 4) with normoactive deep tendon reflexes. She also had bulbar symptoms such as dysarthria, dysphagia, and dysphonia accompanied by jaw weakness. Initial brain magnetic resonance imaging (MRI) showed normal fluid-attenuated inversion recovery (FLAIR) imaging (Fig. 1A) with sulcal enhancement in cortical sulcus and cerebellar folia on FLAIR enhancement study (Fig. 1B). Laboratory findings showed leukopenia (3,000/mm3), normal platelet count (211,000/mm3), increased erythrocyte sedimentation rate (56 mm/hr), normal C-reactive protein level (<0.3 mg/dL), negativity for antiphospholipid antibodies, anti-dsDNA antibody negativity, and normal complement level (C3, 94 mg/dL; C4, 19.4 mg/dL), indicating rather low disease activity. CSF analysis revealed normal opening pressure (120 mmH2O), increased white blood cell count (9/mm3), elevated protein level (93 mg/dL), and lower glucose level (69 mg/dL) compared to serum glucose level (126 mg/dL). Under the suspicion of aseptic meningitis due to SLE, methylprednisolone was started at a dose of 500 mg/day; however, 2 days after starting methylprednisolone, her tremor and the weakness of the extremities worsened, and she was treated with intravenous immunoglobulin (2 g/kg/day) for 5 days along with steroid. On the fifth day of immunoglobulin administration, her vital signs became unstable; blood pressure increased to 153/129 mmHg, and pulse rate increased to 160 beats per minute without improvement of the neurologic symptoms. Additional brain MRI revealed high signal intensities in the occipital region on T2 and FLAIR imaging, suggesting PRES, as well as focal high signal in the right basal ganglia. Moreover, no basal ganglia lesion was observed on diffusion-weighted imaging and normal angiography. These findings suggested the diagnosis of lupus cerebritis and PRES induced by the disease itself or immunoglobulin (Fig. 2). She received symptomatic treatment for hypertension and tachycardia. Simultaneously, she was treated with two cycles of rituximab (850 mg/day for 8 hours) with an interval of 1 week for the newly developed lesions. Although there was no clear improvement in the patientãs neurological findings after the first cycle of rituximab, the tremor and weakness of the extremities and bulbar muscles gradually improved after the second cycle. Twenty days after the last rituximab treatment, she was discharged with improved neurological and psychiatric symptoms (Fig. 3). At 3-month follow-up, brain MRI showed a complete reversal of the occipital and basal ganglia signals (Fig. 4), and she resumed normal daily activity.
DISCUSSION
Here, we present the case of a young woman with lupus cerebritis and PRES who had been previously diagnosed with SLE. Unlike intravenous methylprednisolone and immunoglobulin, two cycles of intravenous rituximab successfully reversed her neurologic and psychiatric symptoms. Furthermore, her radiologic abnormalities along with neuropsychiatric symptoms were completely reversed 3 months after symptom onset.
Neuropsychiatric involvement in patients with SLE has been directly linked to SLE (primary NPSLE) or to other factors such as medicines, systemic diseases, and concomitant psychiatric disorders (secondary NPSLE) [6]. Although the pathogenesis of primary NPSLE is not fully understood, immune or inflammation-mediated blood brain barrier disruption with active generalized disease (inflammatory NPSLE) and thrombotic cerebrovascular disease with antiphospholipid antibody (thrombotic NPSLE) have been suggested to cause primary NPSLE [7,8]. Nevertheless, it is important to manage neuropsychiatric manifestations in SLE patients because they are associated with increased risk of death and worse functional outcome [9,10]. However, the diagnosis of NPSLE remains challenging even for experienced physicians because there is no specific biomarker for NPSLE. Therefore, researchers have attempted to developed various models or algorithms involving disease activity, imaging techniques, and CSF examinations to assist clinicians in diagnosing NPSLE [11]. Our patient was simultaneously diagnosed with lupus cerebritis (primary NPSLE) and PRES (secondary NPSLE) based on clinical findings, brain MRI, and CSF examination. Although rituximab is considered as second-line therapy for inflammatory NPSLE that shows no clinical response to methylprednisolone [4,12], it was more common and easier to use intravenous immunoglobulin, which is consider as third-line therapy for inflammatory NPSLE, in our hospital. However, in this case, intravenous immunoglobulin was not effective, and it might have induced PRES. Nevertheless, we anticipated that immunotherapy against lupus cerebritis and symptomatic treatment with blood pressure lowering for PRES would reverse the patientãs neuropsychiatric symptoms, and this proved to be the case.
The treatment of NPSLE is challenging, and no specific guidelines for the treatment of NPSLE have been established. This could be due to the obscure and complex pathophysiology of NPSLE as well as the fact that well-designed clinical studies are limited by small sample sizes [13]. Nevertheless, it is most important to diagnostically decide whether the neuropsychiatric symptoms are caused by primary NPSLE and the dominant category of NPSLE (inflammatory vs. thrombotic). An inflammatory cause is considered in case of young patients , NPSLE occurrence close to the time of SLE diagnosis, increased lupus activity or flare, antiphospholipid antibody-negative patients, worsening neuropsychiatric symptoms, NPSLE relapses, and abnormal results of CSF examination and brain MRI , and immunosuppressive treatment is recommended in such cases [13]. In inflammatory NPSLE, the first-line treatment of choice is high dose intravenous methylprednisolone. A randomized clinical trial demonstrated that intravenous cyclophosphamide (0.75 g/m2) combined with methylprednisolone was more effective than intravenous methylprednisolone alone [14]. However, because cyclophosphamide is difficult to use and has many side effects, physicians generally hesitate to use it. Despite the lack of evidence from randomized clinical trials, B-cell depleting agents (rituximab; an anti-CD20 monoclonal antibody) are widely used to successfully treat refractory NPSLE in patients who do not respond to first-line therapy [15]. In this case, the patientãs clinical symptoms also gradually improved to complete remission after two cycles of rituximab separated by an interval of 1 week (Fig. 4). Her brain MRI findings also improved 3 months after rituximab therapy.
Accurate diagnosis and treatment of NPSLE are crucial for managing every neuropsychiatric event in patients with SLE. Rituximab may be an effective treatment for refractory inflammatory NPSLE.