INTRODUCTION
Acute intracranial hypertension (AIH) is defined as sustained intracranial pressure (ICP) greater than 20 mmHg for greater than five to ten minutes in a patient that is not being stimulated [1-3]. Many disease processes can result in AIH including traumatic brain injury (TBI), large acute ischemic stroke, and intracerebral hemorrhage. The common denominator in all of these disease processes is the expansion in intracranial volume [3]. Regardless of the cause, AIH is associated with worse outcomes [4]. AIH is a medical emergency that requires rapid diagnosis and treatment to avoid irreversible damage to the brain. Multiple studies have shown improved outcomes and reduced mortality with aggressive treatment of AIH [5-7]. In this review, pathophysiology of ICP, modalities used to monitor ICP, treatment strategies for patients with AIH, and the therapeutic goals of those treatment strategies are discussed in detail.
PATHOPHYSIOLOGY
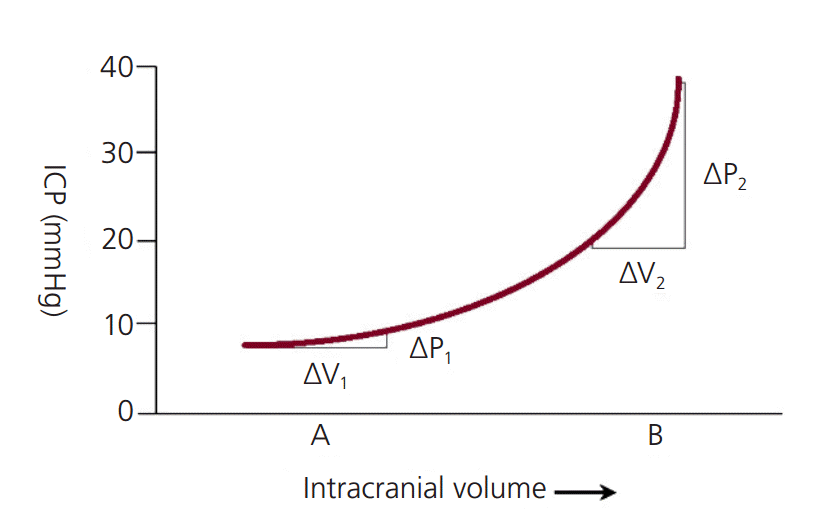
Intracranial compliance
The Monro-Kellie doctrine states that the intracranial space is a fixed volume inside the skull [3]. This intracranial space has three components: brain tissue, blood, and cerebrospinal fluid (CSF). In an average adult, the brain tissue volume is 1,400 mL; the blood volume is 150 mL; and the CSF volume is 150 mL [3]. Normal ICP ranges 3-15 mmHg; in the intensive care unit (ICU), ICP values less than 20 mmHg are generally accepted. Pathologic states within the intracranial vault result in an increase of volume. This is a result of at least one of the following: 1) extrinsic mass lesion, 2) increase in blood volume, 3) increase in CSF volume, or 4) increase in brain tissue. To maintain normal ICP in these pathologic states, there is a reduction in the volume of other compartments as a compensatory mechanism. As this compensatory mechanism reaches its limit, the compliance (delta V/delta P) drastically. As compliance decreases, there is a greater the change in pressure for a given change in volume (i.e., there are dramatic changes in pressure for small incremental changes in volume; Fig. 1). This is reflected in the ICP waveform as seen in Fig. 2.
Cerebral perfusion pressure (CPP) is defined as mean arterial pressure (MAP) minus intracranial pressure (i.e., CPP=MAP-ICP). Therefore as ICP rises, CPP will fall. Normal CPP is 60-150 mm Hg [8]. CPP less than 60 mmHg may result in ischemic brain injury, while CPP greater than 150 mmHg can lead to hyperemia and hyperperfusion injury.
ICP MONITORING MODALITIES
External ventricular drain
An external ventricular drain (EVD) is a catheter inserted into one of the lateral ventricles and connected to an external transducer via tubing filled with saline. In addition to an external transducer, there is also a fluid collection system, and a three-way stopcock allows the clinician to switch between draining fluid and transducing a pressure. The transducer must be positioned at the level of the external auditory meatus (the level of the Foramen of Monro) to accurately measure the intracranial pressure [3]. The system can be set up to continuously drain fluid with intermittent pressure transduction or to continuously transduce a pressure with intermittent fluid drainage. The advantage of an EVD is that it is both diagnostic and therapeutic. Its major disadvantage is the risk of infection leading to meningitis or ventriculitis. Infection occurs on 10-20% of patients and this risk increases after about 5 days [9]. Some catheters are now made with antibiotic coating to help reduce infection rates, but its superiority has not been consistently shown compared to the conventional catheters. Additionally, in some ICUs, it is standard to administer systemic antibiotics during the duration of the time the catheter is in place. In addition to acquiring actual pressure readings, it is also important to pay attention to the ICP waveforms. The second peak of the waveform either at or above the level of the first peak suggests poor brain compliance. When brain compliance is poor – even if the ICP is normal- it is not recommended to put patient’s head down flat (e.g. while obtaining CT or MRI images) or wean sedation as it does not take much for ICP to rise above the acceptable level.
Intraparenchymal pressure monitor
Intraparenchymal pressure monitors are placed into the brain tissue via a burr hole and secured using bolt. A pressure transducer is incorporated directly into the catheter as opposed to an external transducer as discussed with an EVD. The catheter requires calibration once prior to placement. The accuracy of the ICP measurements is generally better than what is achieved with subarachnoid or epidural monitors, which have fallen out of favor [10]. One advantage of Intraparenchymal monitors is ease of insertion as one does not need to aim for a particular space as is required with an EVD. A second advantage is a drastically lower infection rate, which on average is around 1% [11]. The major disadvantage to this modality is that it only allows for ICP monitoring without a combined therapeutic option of CSF drainage. More recently, there are intraparenchymal pressure monitoring catheters that also contain EVDs and other ports for multimodality probes such as brain tissue oxygen or blood flow catheters.
TREATMENT OPTIONS FOR ACUTE INTRACRANIAL HYPERTENSION
Many treatment options exist to treat acute intracranial hypertension. The goal of these therapies is to decrease ICP to less than 20mm Hg; however, the most recent TBI guidelines from the Brain Trauma Foundation (BTF) suggest that the ICP goal should be less than 22 mmHg [12]. A step-wise algorithm is presented here which provides a standardized approach to the management of AIH. While multiple steps can be instituted as once, no step should be skipped before the initiation of a new step. Furthermore, weaning of therapies should occur in a step-wise fashion as well to avoid rebound ICP crises. Use of a stepwise algorithm in an ICU setting not only allows for wellcoordinated treatment of patients, it also can help improve outcomes. A retrospective study suggests that TBI patients in whom ICP was treated with using a standardized algorithm had fewer interventions and shorter duration of treatment compared to patients treated ad hoc [3,13]. Prior to proceeding through the algorithm, a set of general measures should be considered. These include elevation of the head of bed to 30°, position of the head in a midline position, initiation of seizure prophylaxis, volume resuscitation, avoidance of hyperglycemia and hyperthermia.
Step 1. Surgical decompression
The initial step in the management of AIH should always be consideration of whether a surgical intervention such as ventriculostomy or craniectomy with duraplasty should be performed to remove CSF volume or decompress the skull, respectively. Additionally a computed tomography (CT) scan of the head should be obtained to evaluate for increased size of hemorrhage worsening hydrocephalus. If an EVD is already in place, then it should be open to drain CSF. After placement of an EVD, it would be important to always make sure its patency.
For TBI patients, following the initial resuscitation, early (<24 hours) decompressive craniotomy with evacuation of a focal mass lesion is the single most important treatment resulting in improved outcomes [8,14].
For patients with large hemispheric ischemic strokes, decompressive craniectomy in patients with clinical and radiographic evidence of malignant edema reduces ICP and improves outcome [8,15-18]. Predictors of poor outcome after decompressive craniectomy for ischemic stroke include age >60 years, preoperative GCS <8, preoperative anisocoria, early clinical deterioration (<72 hours from stroke onset), and multiple territory infarction [8,19,20].
In patients with intracerebral hemorrhage (ICH), many case series have shown positive results with decompressive craniectomy [8]; however, a large randomized controlled trial failed to demonstrate improved outcomes when decompressive craniectomy was compared to aggressive medical management [21]. Newer surgical techniques using stereotactic and endoscopic clot thrombolysis have shown benefit over medical management alone and randomized controlled trials are currently ongoing [22-25]. Currently surgery is advocated in ICH patients with GCS 6-12, deteriorating neurological examination, lobar ICH, infratentorial ICH greater than 3 cm in diameter (or those hemorrhages less than 3cm in diameter but with significant mass effect with brainstem compression), ventricular obstruction or extension, hydrocephalus, cerebellar vermis involvement, superficial hemorrhage, clot volume 20-80 mL, relatively young patients, and hemorrhage resulting in midline shift or increased ICP [8,26-28]. While decompressive craniectomy can be lifesaving on patients with AIH, it has varying effects on long-term functional outcome.
Step 2. Sedation
Adequate sedation is an often-overlooked therapy in the treatment of AIH. It is not uncommon to see patients in neuro-ICU with high ICP and submaximal dose of sedating medications. Patients with agitation and ventilator dyssynchrony result in increased intrathoracic pressure leading to decreased venous return from the head and increased ICP. Furthermore, agitation raises the systemic arterial pressure, which can result in increased ICP in patients on the extreme of their autoregulatory curve. For these reasons, agitated patients with elevated ICP should be sedated to a quiet motionless state. Additionally, they should not undergo neurologic examinations every 10 minutes in which noxious stimuli can raise the ICP. Choice of sedatives should focus on short-acting agents such that they can be stopped quickly when a neurological examination is necessary. Generally first-line agents include a sedativehypnotic and an analgesic agent. In patients that are hemodynamically stable with euvolemic intravascular volume state, propofol in bolus doses followed by a continuous intravenous infusion can provide adequate sedation. It is not uncommon to see serious hypotension that occurs in IV propofol infusion particular if given to elderly patients who are volume depleted. Given that pain is often a contributor to elevated ICP, especially in TBI patients, the addition of fentanyl can work synergistically with propofol to reach the sedation goal. However, caution should be taken with bolus injections of opioids one can have paradoxical rises in ICP following a bolus injection of an opiate. This occurs because the opioid bolus transiently lowers the MAP which in turn raises the ICP as the cerebral blood vessels dilate to maintain cerebral blood flow (CBF). This usually occurs on patients with an intact autoregulatory system; however, it has been seen in patients with compromised autoregulation as well [3,29,30]. Additionally, propofol may have some neuroprotective properties in TBI patients. One study looking at propofol versus morphine in severe TBI patients showed that the propofol treated patients had better ICP control and more favorable long-term outcomes [3,31]. In hemodynamically unstable patients (i.e., hypotension, poor cardiac output, intravascular volume depletion), midazolam may be a better option given its limited effects on the heart rate and blood pressure.
Step 3. Cerebral perfusion pressure optimization
At the lower spectrum of the autoregulatory curve, the CPP is low and there is a compensatory dilation of the intracerebral arterioles, which increases blood volume and subsequently increases ICP. In this scenario, augmentation of the MAP with a vasopressor medication (e.g., phenylephrine, norepinephrine) will raise the CPP leading to mild arteriolar vasoconstriction with decreased blood volume and decreased ICP. It is unclear what the lower limit of CPP is below which, ICP begins to rise. This varies patient to patient and is generally higher in patients with chronic hypertension where the autoregulatory curve is shifted to the right. In TBI patients, the BTF recommends systolic blood pressure (SBP) ≥100 mmHg for patients aged 50-69 years and SBP ≥110 mmHg for patients aged 15-49 years or >70 years [12]. Their CPP goal recommendation is between 60 and 70 mmHg [12].
Alternatively, if MAP and ICP are elevated in a sedated patient, mild reduction in the MAP may lead to a reduction in the ICP as well. If MAP is >110 mmHg and ICP is >20 mmHg, systemic blood pressure should be carefully lowered using a short-acting, titratable agent (e.g., labetalol or nicardipine). It is important not to decrease the CPP so much that the intracerebral arterioles dilate resulting in reflex increased ICP as discussed in the previous paragraph. As such, generally nitroprusside is not used to treat systemic hypertension in patients with increased ICP due to the concern for direct intracerebral vasodilation and subsequent ICP elevation.
Step 4. Hyperventilation and vasoconstriction
Hyperventilation results in a respiratory alkalosis with an elevated pH and reduced PaCO2. In the setting of increased ICP, the goal should be to lower the PaCO2 to 30 mmHg, or 25-30 mmHg in extreme cases. This reduction in PaCO2 leads to vasoconstriction of the intracerebral arterials and subsequent reduction in ICP. The peak effect is reached within 30 minutes of starting hyperventilation therapy. Over the next 1-3 hours however, the effect diminishes as the buffering capacity of the CSF compensates [3,32]. Therefore, hyperventilation should only be used transiently as a temporizing measure while alternative treatments are being explored. Furthermore, it is postulated that severe hyperventilation (PaCO2 <25 mmHg) leads to cerebral ischemia due to extreme vasoconstriction of the intracerebral arterioles. One study evaluating TBI patient observed poorer outcomes in patients prophylactically hyperventilated to PaCO2 of 20 mmHg compared to patients ventilated normally [33]. Prolonged, prophylactic, and/or profound hyperventilation are discouraged [8,12]. So while harmful with prolonged use, hyperventilation is life saving in patients with acutely elevated ICP as a temporary therapy [34].
Hyperventilation, although it is listed as “step number 4”, it could be simultaneously initiated as steps 1 through 3 are all being instituted.
Step 5. Osmotherapy
After surgical intervention, adequate sedation and hyperventilation step fail to control ICP and optimize CPP, the next immediate step would be to initiate osmotherapy. IV mannitol 1-1.5 g/kg solution infused over 30 minutes is reasonable and may repeat it every 6 hours as needed in order to achieve the target ICP parameter. The initial dose of IV mannitol-especially in the setting of ICP crisis- should not be under-dosed. It is however, not unreasonable to give less than 1 g/kg for the subsequent mannitol doses. For example, 50-75 gm every 6 hour for a 70 kg man is acceptable after the initial 1.5 gm/kg (e.g. 105 g for a 70 kg man) bolus. Alternatively, one may give IV hypertonic saline (HTS). There are different concentrations available: 2%, 3%, 23.4%, ect. In an acute setting where ICP is unacceptably high with impending brainstem herniation, it makes no sense to start 2% NaCl HTS infusion without any bolus at 50 mL/h. Such therapy would take days to drive up the sodium and patient might not survive for such duration of time. Therefore in emergency situations, it is wise to choose a small volume (hence more rapid infusion) and higher concentration (e.g. 23.4%) given as IV push over 15minutes [35,36]. Not every case requires such an aggressive osmotic therapy. An elderly patient, for whom clinicians and families have decided against decompressive hemicraniectomy, who is still awake with significant mass effect and midline shift from malignant middle cerebral artery infarction might only need mild-moderate osmotic effect in order to avoid herniation. In such situation, it may be reasonable to opt for a less concentration HTS (e.g. 2-3% HTS at 50 mL/h continuous infusion) and “drive up” the sodium over 1-2 days rather over the next 2 hours. A reasonable target serum sodium concentration in above scenario would be 150-155 mEq/L just during the herniation watch period. The decision as to mannitol versus hypertonic saline as the choice of osmotherapy depends on patient’s renal condition and intravascular volume status. For those with normal renal function and euvolemic state, either option would lead to significant reduction in ICP. Typically, mannitol should not be used for patients who are hypotensive with sinus tachycardia due to prerenal dehydration as osmotic diuresis would worsen hypotension.
Step 6. Hypothermia and targeted temperature modulation
It is well known that hypothermia with temperature maintenance at 33 degrees Celsius leads to reduction in ICP. Whether hypothermia is truly neuroprotective or not in brain injured patients is not clear at this time, however. The only class I evidence so far for improving neurological, functional outcomes is the use of hypothermia after out-of-hospital ventricular fibrillation and ventricular tachycardia cardiac arrest [37]. Recently, this data is being reinvestigated after 36 degrees Celsius had the same outcome compared to 33 degrees Celsius [38]. For patients with high ICP, hypothermia has shown consistent findings: it lowers ICP but does not confer improved long term outcomes [39]. Some of these studies skipped essential early step (i.e. osmotherapy) for reducing ICP and hence may not accurately reflect the real life experience of the benefit that hypothermia might provide. Nevertheless, there is no class I evidence that reducing body temperature improves long term outcome. But again, hypothermia does reduce ICP and it may be used to optimize CPP as one of the more aggressive management strategies.
What is more important at the bedside is probably recognizing the limitations and serious adverse events that are associate with hypothermia especially if used for a prolonged period of time. Hypokalemia (relative hypokalemia as the total body potassium level is likely the same- it is the into-the-cell shift leading to a low plasma concentration of potassium), atrial and ventricular arrhythmias, hypotension may occur. The negative hemodynamic effect is not trivial. There may be also tendency for coagulopathy as well as significantly elevated risk of infection, particularly ventilator associated and nosocomial pneumonia [40].
Step 7. Barbiturate coma
The use of pentobarbital coma has clearly fallen out of favor in recent years, and this is due to its inevitable and unacceptably serious side effects. Pupils may become fixed and dilated. Its negative, cardiac suppressive effect leads to severe hypotension and increase in vasopressor requirements. An increase in systemic vascular resistance caused by the use of high dose vasopressors may lead to end organ hypoperfusion and significant metabolic acidosis. A severe metabolic acidosis makes vasopressors to become ineffective and cause refractory hypotension which leads to even further increase in pressor requirements. A vicious cycle like this may lead to cardiac arrest and death. Therefore in routine intensive care unit setting for intracranial hypertension, this medical therapy has either disappeared or remains as one of the last resort therapies. In certain neurosurgical emergencies such as intraoperative rupture of intracranial vessels (e.g. arteriovenous malformation resection surgery with intraoperative rupture) with super refractory ICP, a rapid bolus of IV pentobarbital injection may provide effective reduction in pressure. However, a prolonged use of IV pentobarbital drip is generally not recommended any more.